* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Promoting central nervous system regeneration: Lessons from
Survey
Document related concepts
Transcript
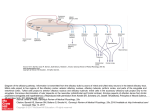
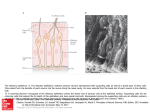
Promoting central nervous system regeneration: Lessons from cranial nerve I Ruitenberg, M., & Vukovic, J. (2008). Promoting central nervous system regeneration: Lessons from cranial nerve I. Restorative Neurology and Neuroscience, 26(2-3), 183-196. Published in: Restorative Neurology and Neuroscience Document Version Publisher's PDF, also known as Version of record Link to publication in the UWA Research Repository Rights statement Link to Publisher's website supplied in Alternative Location. General rights Copyright owners retain the copyright for their material stored in the UWA Research Repository. The University grants no end-user rights beyond those which are provided by the Australian Copyright Act 1968. Users may make use of the material in the Repository providing due attribution is given and the use is in accordance with the Copyright Act 1968. Take down policy If you believe this document infringes copyright, raise a complaint by contacting [email protected]. The document will be immediately withdrawn from public access while the complaint is being investigated. Download date: 16. Jun. 2017 183 Restorative Neurology and Neuroscience 26 (2008) 183–196 IOS Press Promoting central nervous system regeneration: Lessons from cranial nerve I Marc J. Ruitenberg∗ and Jana Vukovic Experimental and Regenerative Neuroscience (EaRN), School of Anatomy and Human Biology (M309), The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia Abstract. The olfactory nerve differs from cranial nerves III-XII in that it contains a specialised type of glial cell, called ‘olfactory ensheathing cell’ (OEC), rather than Schwann cells. In addition, functional neurogenesis persists postnatally in the olfactory system, i.e. the primary olfactory pathway continuously rebuilds itself throughout adult life. The presence of OECs in the olfactory nerve is thought to be critical to this continuous growth process. Because of this intrinsic capacity for self-repair, the mammalian olfactory system has proved as a useful model in neuroregeneration studies. In addition, OECs have been used in transplantation studies to promote pathway regeneration elsewhere in the nervous system. Here, we have reviewed the parameters that allow for repair within the primary olfactory pathway and the role that OECs are thought to play in this process. We conclude that, in addition to intrinsic growth potential, the presence of an aligned substrate to the target structure is a fundamental prerequisite for appropriate restoration of connectivity with the olfactory bulb. Hence, strategies to promote regrowth of injured nerve pathways should incorporate usage of aligned, oriented substrates of OECs or other cellular conduits with additional intervention to boost neuronal cell body responses to injury and/or neutralisation of putative inhibitors. 1. Introduction Functional nervous system regeneration in adult mammals can occur but seems mainly restricted to the peripheral compartments. Within the central nervous system (CNS), regenerative capacity is very limited and injured nerve cells normally fail to regrow an axon to restore communication with other parts of the brain or spinal cord. As a consequence, injury to the CNS usually results in long-lasting behavioural impairments. The development of strategies that could alleviate the permanent consequences of CNS trauma is a major challenge to neuroscience. Reconstruction of central pathways is particularly challenging due to limited intrinsic regenerative potential of CNS neurons, scar formation and the development of cyst-like tissue cavitations. In the latter situation, some form of transplantation will be required to restore tissue continuity and to provide a structural ∗ Corresponding author. Tel.:/Fax: +61 8 6488 7513 (1051); Email: [email protected]. substrate for axons regenerating to the distal neuropil. Various candidate donor tissues and cells have been trialled as biological bridging material, including foetal tissue, peripheral nerve or Schwann cell grafts, olfactory ensheathing cells (OECs) and, more recently, bone marrow stromal and other progenitor (-like) cells (for reviews, see e.g. Bunge and Pearse; 2003; Emsley et al., 2005; Myckatyn et al., 2004; Reier, 2004; Harvey et al., 2006; Nandoe et al., 2006). At first sight, OECs would appear to have a natural advantage over many other cellular transplant materials as they reside within the olfactory nerve layer of the olfactory bulb, which is considered part of the CNS, and normally co-exist with astrocytes (Doucette, 1991; 1993). Also, since the primary olfactory pathway continuously rebuilds itself throughout adult life, these specialised glial cells seem ideal candidates for supporting axon tract regeneration following transplantation into other areas of the CNS. Indeed, several pioneering studies using OEC transplants in CNS injury paradigms claimed significant regeneration (RamonCueto et al., 1994; Li et al., 1997, 1998; Ramon-Cueto et al., 1998, 2000). These studies generated much ex- 0922-6028/08/$17.00 2008 – IOS Press and the authors. All rights reserved 184 M.J. Ruitenberg and J. Vukovic / Promoting central nervous system regeneration citement amongst scientists and the broader community as a potential treatment for CNS trauma that could, at least partially, end the permanence of related disabilities. However, other laboratories have been unable to reproduce some of the effects reported in the initial in vivo studies, obtained mixed results in terms of OECmediated regeneration, and clinical trials have yet to show any beneficial effects in spinal cord-injured patients (e.g. Ramer et al., 2004; Riddell et al., 2004a,b; Feron et al., 2005; Lu et al., 2006). The aim of this review is not to provide a complete overview of all studies that have used OECs to promote CNS regeneration as several recent reviews have already extensively covered this topic (Barnett & Riddell, 2007; Franssen et al., 2007; Richter & Roskams, 2007). Instead, we will revisit the parameters that allow for successful regeneration to occur in the periphery, specifically the primary olfactory pathway. We will first discuss the aspects that underlie the continuous successful regenerative events within the first cranial or olfactory nerve and the role OECs are thought to play within this process. We will then proceed to compare the natural repair process in the first cranial nerve to injury responses in the CNS. Identified differences can provide clues as to why the regenerative process might fail in the CNS and how improvements could be made for future repair strategies. 2. Plasticity and repair in the primary olfactory pathway 2.1. Natural regenerative events The primary olfactory pathway, consisting of the olfactory neuroepithelium, nerve and bulb, retains a remarkable potential for self-repair throughout adult mammalian life. The adult olfactory epithelium (OE), which lines the cartilaginous turbinates in the caudal regions of the nasal cavity, seems to persist in a continuous state of olfactory sensory neuron (OSN) turnover (e.g. Harding et al., 1977; Graziadei et al., 1979; for review, see Schwob, 2002). The preserved potential for self-repair within the primary olfactory pathway makes sense when taking into account the vulnerability of OSNs to environmental insults and importance of the sense of olfaction to most vertebrates. The OE itself is usually divided into three regions, i.e. basal, intermediate and apical (see Fig. 1). The top or apical layer of the OE consists of mucus, the ciliated endings of OSN dendrites, and the cell bodies of sustentacular and microvillar cells. The intermediate or middle compartment of the epithelium contains the OSNs, which are typical bipolar nerve cells. In addition to the apical dendrite that projects to the luminal surface of the OE, each OSN also gives rise to a single axon that leaves the epithelium through gaps in the basal lamina and projects to the main olfactory bulb via the olfactory nerve. At the level of the olfactory bulb, these axons terminate in appropriate glomeruli where they synapse on second-order neurons. A precise topographic map exists at the level of the olfactory bulb based on odorant receptor expression (Wang et al., 1998) and activity-based refinement of connections (Zou et al., 2004). Both mature and immature OSNs reside in the intermediate layer of the OE. The life stage of OSNs can be indentified based on expression of olfactory marker protein (OMP) for mature OSNs (Margolis, 1972; Farbman & Margolis, 1980), and β 3tubulin or growth-associated protein B-50/GAP-43 for immature OSNs (Verhaagen et al., 1989; Lee & Pixley; 1994). These immature OSNs are the progeny of horizontal and globose basal cells, which make up the stem cell/progenitor niche of the OE (Harding et al., 1977; Graziadei & Monti-Graziadei, 1979; Caggiano et al., 1994; Jang et al., 2003; Leung et al., 2007). Although it is accepted that mature OSNs are being turned over in the adult OE, data on their average lifespan in the normal OE is less clear. A number of original studies have put forward an average turnover rate of approximately 4 weeks (e.g. Moulton, 1974; Graziadei & Monti-Graziadei, 1979). However, others have challenged this view and suggested this turnover might be much slower with some receptor cells in mice living up to 12 months of age, i.e. to at least half the average life expectancy of this species (Hinds et al., 1984; Mackay-Sim & Kittel 1991a, b). The majority of adult-born cells in the OE do not appear to mature or survive long-term and most undergo apoptotic death within weeks of being born (Mackay-Sim and Kittel, 1991a; Carr & Farbman, 1993; Deckner et al., 1997) as they probably fail to integrate appropriately into existing circuitry. Hence, under normal circumstances, a small number of cells entering the apoptotic pathway can always be detected in the OE. Apoptosis seems to be the default pathway of cell death in the OE (Cowan et al., 2001). Dying cells are rapidly cleared by phagocytosis (Suzuki et al., 1995) and substituted by new odorant receptor-expressing cells from the pool of immature OSNs and basal progenitors. Molecular signals derived from OSNs, their progenitors and epithelial macrophages have been implicated in the con- M.J. Ruitenberg and J. Vukovic / Promoting central nervous system regeneration 185 Fig. 1. Organisation of the olfactory epithelium and the underlaying lamina propria. A. Schematic drawing of the olfactory epithelium (OE) and lamina propria. The OE constitutes a number of cell types. Basally, horizontal and globose basal cells can be found which form the germinal zone of the OE and give rise to olfactory sensory neurons (OSNs). The intermediate compartment contains both mature and immature OSNs, which can be identified via expression of olfactory marker protein (OMP; red) or β 3-tubulin (green), respectively. Most superficially, the sustentacular and microvillar cells can be found. These cells are thought to play a role in general support of OSNs and their apical dendrites. Axons of mature and immature OSNs exit the OE through gaps in the basement membrane. In the lamina propria, bundles of OSN axons are enwrapped by olfactory ensheathing cells (OECs). The lamina propria also contains blood vessels as well as a number of fibroblast further enveloping the nerve bundles. B, C: Confocal images of immunostained sections of intact and Triton-X lesioned mouse OE. An area similar to that depicted in A is shown. The intact olfactory epithelium (B) exhibits a large population of mature OMP+ OSNs with relatively few TUJ1+ positive cells. After injury (C), a loss of OMP+ OSNs and significant thinning of the OE can be observed. Eventually, the OE will recover and the OMP+ layer repopulated from immature OSNs and basal progenitors to control levels as shown in (B). A color print of this figure is available in the online version of the article. A color print of this figure is available in the online version of the article. 186 M.J. Ruitenberg and J. Vukovic / Promoting central nervous system regeneration trol of neurogenesis in the OE (Kawauchi et al., 2004; Borders et al., 2007). Finally, it is important to keep in mind that all of the above findings on OSN turnover are based on observations made in laboratory mice that are usually kept under very clean and controlled conditions. As suggested by Hinds et al., (1984), rhinitis and other forms of irritation and inflammation of the nasal cavity can dramatically alter the rate of OSN turnover; thus the dynamics of cell death and replacement in the OE of mice and other species in the wild might be very different. 2.2. Experimental injury and olfactory nerve regeneration Because of its intrinsic capacity for ongoing selfrepair, the primary olfactory pathway has proven an attractive model to study nerve cell replacement and pathway regeneration in the mature nervous system. Several lesion models have been developed to study the cellular and molecular aspects that govern the regenerative events in the OE and nerve. These interventions employ techniques that either ablate or deafferent the olfactory bulb and induce rapid (retrograde) death of OSNs, usually via the apoptotic pathway (Cowan et al., 2001). Both macrophages and sustentacular cells have been implicated in the phagocytic clearance of dead cells and debris from the OE (Suzuki et al., 1995; 1996). All experimental models induce en mass OSN turnover and de novo axonal growth but the final outcome may vary. Olfactory bulbectomy induces a robust regenerative response from the basal OE progenitor compartment but the epithelium remains thinner and never fully recovers (Costanzo and Graziadei,1983; Verhaagen et al., 1990). The latter phenomenon is most likely explained by a new state of epithelial tissue homeostasis in the absence of synaptic targets. Deafferentation of the olfactory bulb is usually achieved via: 1) surgical intervention involving olfactory nerve transection (Doucette et al., 1983) or 2) epithelial lesions using gaseous or soluble olfactory toxicants (Matulionis, 1975; Verhaagen et al., 1990; Schwob et al., 1995; Bergman et al., 2002). Recently, Chen et al., (2005) reported the development of an additional lesion model via generation of a transgenic mouse in which the human diphtheria toxin receptor is expressed under control of the OMP promoter, i.e. on mature OSNs. This model offers an additional and controlled method to specifically ablate mature OSNs for the study of peripheral neurogenesis and regeneration of the olfactory epithelium and nerve. In the presence of a target structure, epithelial recovery and pathway reconstruction following chemical deafferentation in mice is largely achieved after 6–8 weeks (e.g. Cummings et al., 2000). 2.3. Responses of OECs to axonal loss and injury En route to the glomerular layer of the olfactory bulb, the axons of OSNs associate with OECs. These specialised glial cells are present within peripheral olfactory nerve fascicles as well as in the centrally-located olfactory nerve fibre layer (ONL) that envelops the olfactory bulb. In contrast to other areas of the nervous system, no glia limitans is present at the transition zone or interface where olfactory nerve fascicles enter or merge with the nerve fibre layer of the olfactory bulb, i.e. a direct continuum exists between the peripheral and central nervous system (Doucette, 1991). Within the bulb, the ONL itself can be further subdivided into an outer and inner layer. Although OECs are present in both ONL sublayers, those expressing low-affinity nerve growth factor receptor, p75, are mainly located in the outer layers of the ONL whereas p75-negative, neuropeptide Y-positive OECs make up the inner layer (Ubink et al., 1994; Ubink and H ökfelt, 2000; Au et al., 2002). Distribution of glial marker S-100 is relatively uniform in both layers but the relationship between OECs and OSN axons is less orderly in the inner part of the ONL. As suggested by Au et al., (2002), this different cytoarchitecture within the ONL probably serves distinct functions, i.e. axonal growth and extension in the outer layers while sorting and glomerular targeting occurs in the more inner layers. It is unclear at present whether the antigenic differences observed in situ are the result of differential organisation of the ONL or if they, together with lamina propria-derived OECs, represent true sub-populations of OECs (e.g. Au & Roskams, 2003; Richter et al., 2005). Although OECs have a different developmental origin compared to other macroglia (Chuah & Au, 1991), transcriptome analysis has shown that they display many similarities to both Schwann cells and astrocytes (Vincent et al., 2005a). Resemblance between Schwann cells and OECs also exists in response to injury. Similar to Schwann cells in regenerating peripheral nerve (Heumann et al., 1987), the expression of low-affinity nerve growth factor receptor p75 is re-induced in OECs after injury and loss of axonal contact (Turner & Perez-Polo, 1993; Gong et al., 1994; Turner & Perez-Polo, 1994; Wewetzer et al., 2005). Although the role of p75 regula- M.J. Ruitenberg and J. Vukovic / Promoting central nervous system regeneration tion during regeneration is not precisely understood, studies on knock-out mice have suggested that signalling through this neurotrophin receptor influences growth of OSN axons (Tisay et al., 2000). Also, OECs were demonstrated to have strong phagocytic activity in vitro (Wewetzer et al., 2005), suggesting that, like Schwann cells, they may actively participate in clearance of cellular debris during nerve regeneration. However, unlike the proliferative and migratory responses of Schwann cells after peripheral nerve crush or transection (Fawcett & Keynes, 1990), independent studies have claimed the absence of OEC division and migration in response to axonal degeneration in the olfactory nerve (Williams et al., 2004a; Williams et al., 2004b; Li et al., 2005). Instead, OECs maintained open channels that allowed for de novo OSN axonal growth. As the continuity of the aspects of olfactory nerve investigated in these studies was not disrupted, it is difficult to assess whether these observations represent true differences between Schwann cells and OECs in response to injury. An earlier study by Schwob et al., (1994) shows clear evidence of OEC migration following olfactory nerve transection or bulbectomy, i.e. disruption of pathway continuity. Together with several in vitro studies that demonstrated a high degree of OEC plasticity and motility (van den Pol & Santarelli, 2003; Vincent et al., 2005b; Windus et al., 2007), these findings suggest that OEC migration is likely to occur following mechanical nerve injury or shearing. 2.4. A role for OECs in supporting neurite growth Although epithelial progenitors and immature OSNs have a high intrinsic growth potential, several lines of evidence also suggest an important role for OECs in supporting axonal elongation. During development, olfactory axons already associate with these glial cells from a very early stage and OSN growth cones do not seem to move ahead of migrating OECs (Tennent & Chuah, 1996). Olfactory neurites in vitro preferentially grow on p75-positive OECs (Ramon-Cueto et al., 1993), and this interaction actively supports their growth (Kafitz & Greer, 1999; Tisay & Key, 1999). Molecular characterisation has shown that OECs express a variety of growth-promoting extracellular matrix molecules as well as soluble neurotrophic factors (Kafitz & Greer, 1999; Tisay & Key, 1999; Boruch et al., 2001; Woodhall et al., 2001; Wewetzer et al., 2001; Lipson et al., 2003; Moreno-Flores et al., 2003; Au et al., 2007). Two studies by Hayat et al., (2003a, b) showed that Ca2+ levels and signalling in OECs are 187 important for their support of axonal growth. Recently, Rieger et al., (2007) demonstrated a direct link between olfactory nerve activity and Ca 2+ levels in OECs, providing a mechanism for axon-glia communication and how this may influence growth. Finally, the growthpromoting properties of OECs in vitro are not restricted to olfactory neurons as numerous reports have shown stimulating effects of these glial cells on neurite formation from other neuronal populations, suggesting that could be useful in supporting growth elsewhere (Sonigra et al., 1999; Hayat et al., 2003a; Hayat et al., 2003b; Moreno-Flores et al., 2003; Chung et al., 2004; Deumens et al., 2004; Deumens et al., 2006b; Leaver et al., 2006; Pastrana et al., 2006; Pastrana et al., 2007; Pellitteri et al., 2007). It is of interest to mention that, regardless of the potential for complete self-repair, several case reports have detailed persistent olfactory dysfunction and failed or incomplete regeneration of the olfactory nerve due to scar formation and/or collapse of foramina following severe head trauma (e.g. Reiter et al., 2004). The anecdotal “surfers’ smell” can have a double meaning in that persistent olfactory deficits are relatively common following board impact injuries to the head; the latter causing shearing of olfactory nerve endings from the olfactory bulb as the brain moves within the anterior cranial fossa with no return of smell. Careful analysis of available literature reveals that aberrant innervation and incomplete regeneration also occurs in mice, in particular following surgical transection of the olfactory nerve (Costanzo, 2000; Christensen et al. 2001). In fact, failed regeneration attempts reportedly can cause neuroma formation in the absence of a structurally intact pathway or aligned substrate (Schwob et al. 1994). Also, for ultimate restoration of appropriate glomerular reinnervation and maintained rhinotopy, sparing of a substantial number of fibres expressing the same odorant receptor is very important (John & Key, 2003; for review, see Schwob, 2002). Taken together, these findings demonstrate that structural pathway integrity is a key contributor for successful regeneration of the olfactory nerve following chemical deafferentation. Immature OSN axons use the existing nerve scaffold to grow and appropriately connect with the olfactory bulb (Schwob, 2002). On the other hand, olfactory nerve transection and bulbar injury can cause scar formation and structural disorganisation of the olfactory nerve layer, which impedes with the regeneration process (see Fig. 2). From the above, several important characteristics have emerged for repair in the primary olfactory path- 188 M.J. Ruitenberg and J. Vukovic / Promoting central nervous system regeneration Fig. 2. Schematic representation of possible outcomes foll owing experimentally induced regeneration of the olfactory epithelium (OE). A: Exposure of olfactory sensory neurons (OSNs; arrows) in the nasal cavity to olfactory toxicants results in their subsequent death and replacement. A new wave of OSNs differentiate from the globose cell layer and grow an axon to wards their target structures (glomeruli) in the olfactory bulb. As the original pathway is not perturbed, axons from new-born OSNs arrive at their designated glomerulus (open arrowheads) and the original pattern of innervation is restored. B: Mechanical injury or olfactory nerve transection (red dashed line) can compromise pathway integrity and even cause scar tissue formation. e.g. within foramina of the cirbirform plate. In this scenario, the regenerative events can lead to several possible outcomes. First, OSN axons succeed in their passage through the cribriform plate and reconnect with their correct tanget glomeruli (open arrows) However,aberrant innervation can also been observed (1). Second, should OSN axons encounter a barrier in tge form of scar tissue, neuroma formation (e.g. in front of blocked foramina in the cribriform plate) can occur (2). Testimony to the high intrinsic growth potential of immature OSNs, their axons can turn around when confronted with a scar and grow back into the OE. In the latter situation, intraepithelial neuromas can from (3). These three adverse outcomes appear directly related to the fact that integrity of the primary olfactory pathway was affected by the type of the injury. A color print of this figure is available in the online version of the article. A color print of this figure is available in the online version of the article M.J. Ruitenberg and J. Vukovic / Promoting central nervous system regeneration way and regenerative attempts elsewhere in the nervous system. First, regeneration in the primary olfactory pathway occurs at a system level, i.e. injured OSNs do not regenerate their axons but rather die and the pathway itself rebuilds from an endogenous progenitor pool. However, as regeneration can occur elsewhere in the periphery, it seems that the intrinsic cellular growth potential in response to injury rather then progenitor presence is a prerequisite for repair. Second, the first cranial nerve does not have an astrocytic glia limitans and, although astrocytes are present in the olfactory nerve layer (Doucette, 1991), a recent study by O’Toole et al., (2007) suggests that the presence of OECs might have a dampening effect on astrogliosis. The absence of a classic glia limitans might be an important difference to other peripheral parts of the nervous system, e.g. sensory (dorsal) rootlets in the spinal cord. Here, regenerative attempts of dorsal root ganglion neurons after crush injury usually stall at the level of the dorsal root entry zone where regrowing axons encounter astrogliosis and upregulated expression of inhibitory molecules (Zhang et al., 2001). Third, pathway continuity is normally maintained during successful regenerative attempts within the primary olfactory projection. This feature seems another key requirement for repair. Even though peripheral nerve injuries elsewhere usually will compromise pathway continuity in some form, Schwann cells within the nerve will respond via proliferation, dedifferentiation and the formation of B ünger bands; the latter providing an aligned substrate for regrowth. However, similar to the olfactory nerve, a failure to do so can result in neuroma formation which is detrimental for regeneration. 3. CNS injury and cellular responses Judging by these three repair parameters, regeneration attempts in the CNS fail at least at two levels. Cellular responses (or lack thereof) in the lesioned CNS differ from peripheral injuries at both the neuronal and glial level. CNS neurons usually undergo retrograde cell death or marked atrophy in response to axotomy. When deprived of a larger proportion of their collaterals, neurons are often subject to death (e.g. Fry & Cowan, 1972; Giehl & Tetzlaff, 1996). On the other hand, if the lesion occurs far enough away from the cell body, compromised neurons might survive the axonal injury but become atrophic as a consequence of connectional loss with their targets. Accordingly, projection neurons in the brain develop severe retrograde atrophy but do 189 not necessarily die following spinal axotomy (Kwon et al., 2002; Hains et al., 2003; Ruitenberg et al., 2004). Interestingly, some growth potential seems to persist in the CNS as regeneration-associated gene expression can be temporarily induced by lesioned neurons but this response ceases with the progression of atrophy (Tetzlaff et al., 1991). In addition, this response is generally weaker when compared to regenerating peripheral neurons and its induction is dependent on the distance of axotomy relative to the cell body (e.g. Tetzlaff et al., 1994; Fernandes et al., 1999; Mason et al., 2003). Spontaneous regrowth of some adult rat retinal ganglion cell axons after central visual pathway lesions has been reported (Harvey & Tan, 1992), but in general distal injuries do not evoke the desired regenerative response in the axotomised neuron and regrowth attempts of lesioned nerve fibres will be marginal at best, even in the presence of an aligned substrate (e.g. Kobayashi et al., 1997). In the latter situation, augmenting the neuronal cell body response to injury, e.g. via delivery of neurotrophins, significantly improves the number of regrowing axons (for reviews, see Plunet et al., 2002; Harvey et al., 2006). At the injury site itself, the severed axon stump is faced with pathway discontinuity and does not encounter an aligned glial substrate. Astroglial cells respond to injury by upregulating glial-fibrillary acidic protein (GFAP) and accumulation at the lesion border, i.e. delineating the core of the lesion. This astrocytic response helps to contain the primary injury and likely protects spared CNS tissue from secondary degeneration but is unfavourable for regrowth. While the injury zone is being cleared from cellular debris by phagocytosis, other cells such as meningeal fibroblasts can invade the injury site. Together with hypertrophic astrocytes, these cells form the major constituents of the gliofibrotic scar. This neural scar tissue has a chaotic, disorganised appearance and several inhibitory molecules and guidance cues have been associated with it (McKeon et al., 1991; Pasterkamp et al., 1999; De Winter et al., 2002; Bundesen et al., 2003; Goldshmit et al., 2004; Wehrle et al., 2005). Thus, even if regeneration would be attempted the process is likely to fail due to poor neuronal responses and the absence of a directional growth substrate with appropriately balanced and oriented guidance cues. The formation of aberrant sprouts and ramifications from some axon stumps (Ramon Y Cajal, 1928, 1991) is further indicative that an aligned substrate is important to prevent disorganised and aberrant growth and maintain topographic arrangements if regrowth is attempted (see also Stokols & Tuszynski, 2006). 190 M.J. Ruitenberg and J. Vukovic / Promoting central nervous system regeneration 4. OEC transplants to promote neuroregeneration The natural co-existence of OECs with astrocytes make them attractive candidates for cell-based transplantation strategies that aim to promote axon regrowth in the CNS. Few studies have directly combined or compared the effects of Schwann cell and OEC transplants in regeneration studies (Ramon-Cueto et al., 1998; Takami et al., 2002; Cui et al., 2003; Pearse et al., 2004; Fouad et al., 2005; Barakat et al., 2005; Moon et al., 2006; Andrews & Stelzner, 2007; Pearse et al., 2007; Vavrek et al., 2007; Vukovic et al., 2007). In general, grafted Schwann cell appeared to survive better in comparison to OECs (Cui et al., 2003; Barakat et al., 2005; Pearse et al., 2007; Vukovic et al., 2007) and their presence resulted in more axonal regrowth (Takami et al., 2002; Cui et al., 2003; Barakat et al., 2005) and better protection for spared CNS tissue against secondary degeneration and/or functional recovery (Takami et al., 2002; Moon et al., 2006). Even though mature Schwann cells are potent promoters of CNS axon growth (Richardson et al., 1980; David & Aguayo, 1981; Takami et al., 2002), compelling evidence suggests that their presence is not well tolerated within the CNS. In comparison to OECs, Schwann cell grafts may entrap regenerating axons (Xu et al., 1997), interfere with target innervation (Vukovic et al., 2007) and induce host astrogliosis (Plant et al., 2001; Lakatos et al., 2003; Andrews & Stelzner, 2007). In contrast, transplanted OECs freely interact with host astrocytes without increasing hypertrophy and scarring (Lakatos et al., 2003). This feature of OECs has been put forward as crucial and fundamental to their ability to support regenerative growth across injury sites (for review, see Li et al., 2005; Raisman & Li, 2007). However, the assumed advantage of being able to intermingle with other cell types may come at a price as identified OECs do not naturally form a clustered and aligned substrate across CNS injury sites. Instead, OECs rather adapt to the (developing) cytoarchitecture of the transplantation site and disperse and orientate themselves accordingly (Ruitenberg et al., 2002; Boyd et al., 2004; Ramer et al., 2004; Pearse et al., 2007; Vukovic et al., 2007). The intermingling feature of OECs could potentially be advantageous in small lesions, e.g. root avulsions, if OEC presence following root anastomosis creates passages for regenerating axons to cross the astrocytic barrier (Li et al., 2004; Li et al., 2007). However, larger lesions with both scar and cyst formation will likely require an aligned, oriented substrate to allow for re- growth of white matter tracts. Recent attempts to provide lesioned CNS axons with an oriented OEC substrate showed little improvement in regeneration (Deumens et al., 2006a). However, as discussed earlier, it is possible that regeneration was abortive or failed at the first level, i.e. an inadequate intrinsic regenerationassociated response in the neuronal populations investigated as a ten-fold increase in neurofilament-positive axons was seen in these grafts. Poor survival of transplanted OECs may also have been a confounding factor (Barakat et al., 2005; Pearse et al., 2007; Vukovic et al., 2007). Further insights into molecular factors that determine whether a cell can freely interact with astrocytes (without loosing its bridging capabilities) are also needed (e.g. Fairless et al., 2005; Santos-Silva et al., 2007) and may open possibilities for cellular manipulation to promote regeneration beyond the injury site. Finally, many regeneration studies in the spinal cord have reported beneficial effects of OEC transplants on functional recovery even though no anatomical basis for this seemed present (for a complete overview, see Franssen et al., 2007; Richter & Roskams, 2007). As these experimental injuries are rarely anatomically complete, a number of factors may have contributed to this effect, i.e. tissue sparing, remyelination of denuded axons, local compensatory sprouting or plasticity, etc (e.g. Plant et al., 2003; Ruitenberg et al., 2003; Chuah et al., 2004; Sasaki et al., 2004; Sasaki et al., 2006; Toft et al., 2007). Since these effects are not related to tract repair, it is likely that they are not specific to OECs and could also be achieved with other cellular conduits. 5. Summary The relevance for transplanting OECs at sites of injury outside the primary olfactory pathway has often been based on the fact that the presence of these specialised glial cells is a key difference between a regenerating system such as the olfactory bulb and the rest of the CNS. Based on the first part of this review, one could question the accuracy of such a hypothesis. Although the presence of OECs probably supports olfactory axon growth, a view rather different from ‘constant OEC-mediated olfactory nerve repair’ can be adopted; this is, continuous generation of immature OSNs from basal progenitors occurs but the long-term survival and maturation of the former is critically reliant on available synaptic space. In this respect, the neurogenic events in the OE would display a striking similarity M.J. Ruitenberg and J. Vukovic / Promoting central nervous system regeneration to the other two main areas of adult neurogenesis, i.e. the hippocampus and subventricular zone, where newborn neurons also seem to depend for their survival on incorporation into neuronal networks (Kempermann et al., 1998; Whitman & Greer; 2007). Data from studies on regeneration of the olfactory epithelium and nerve after experimental injury suggests that structural and biochemical integrity of the pathway is of equal importance to high intrinsic neuronal growth potential and crucial for an optimal outcome. Even though immature OSNs have a high intrinsic potential for growth, they need the existing scaffold of other nerve fibres and OEC processes to restore connections with the olfactory bulb (Schwob, 2002). Similar to other areas of the nervous system, repair processes in the first cranial nerve can be suboptimal or even fail if its continuity is compromised and/or scar formation is encountered. With the presence of a directional scaffold as an additional key requirement for repair, the principal findings of Magavi et al. (2000) and Chen et al., (2004) are particularly interesting. Using specific laser-induced apoptosis of corticothalamic and corticospinal projection neurons, the authors showed that even CNS pathways can be restored from endogenous neural progenitors as long as the trajectory to the target area is not compromised. Similarly, microtransplanted DRG neurons display vigorous growth within CNS white matter tracts (Davies et al., 1997) but fail to regenerate axons across the injury site due to glial scarring and absence of an appropriately-oriented substrate (Davies et al., 1999). To improve the functional outcome following lesions that are anatomically incomplete, probably the most feasible and promising interventions lie in the containment of secondary damage and activation of dormant compensatory mechanisms from spared components. The promotion of damaged nerve pathways to 1) regrow their severed axon stumps and 2) appropriately re-connect with distal target regions will likely prove a great deal more difficult. In the quest for optimal design of strategies to promote white matter tract regeneration, future studies should adopt a multifactorial approach that combines enhancement of the neuronal response to injury with neutralisation of inhibitory factors and the provision of a substrate that allows for directional growth (for review, see Plunet et al., 2002; Harvey et al., 2006; Pearse & Bunge, 2006; Oudega, 2007). Acknowledgements Marc Ruitenberg is a postdoctoral fellow supported by the Australian Research Council (Grant No. 191 DP0774113). The authors would like to thank their colleague Prof. Alan R. Harvey for valuable discussions and comments on the manuscript. This work was further supported by The Neurotrauma Research Program of Western Australia and School of Anatomy and Human Biology, The University of Western Australia. References Andrews, M. R., & Stelzner, D. J. (2007). Evaluation of olfactory ensheathing and Schwann cells after implantation into a dorsal injury of adult rat spinal cord. J Neurotrauma, 24(11), 17731792. Au, E., Richter, M. W., Vincent, A. J., Tetzlaff, W., Aebersold, R., Sage, E. H., et al. (2007). Sparc from olfactory ensheathing cells stimulates schwann cells to promote neurite outgrowth and enhances spinal cord repair. J Neurosci, 27(27), 72087221. Au, E., & Roskams, A. J. (2003). Olfactory ensheathing cells of the lamina propria in vivo and in vitro. Glia, 41(3), 224-236. Au, W. W., Treloar, H. B., & Greer, C. A. (2002). Sublaminar organization of the mouse olfactory bulb nerve layer. J Comp Neurol, 446(1), 68-80. Barakat, D. J., Gaglani, S. M., Neravetla, S. R., Sanchez, A. R., Andrade, C. M., Pressman, Y., et al. (2005). Survival, integration, and axon growth support of glia transplanted into the chronically contused spinal cord. Cell Transplant, 14(4), 225-240. Barnett, S. C., & Riddell, J. S. (2007). Olfactory ensheathing cell transplantation as a strategy for spinal cord repair – what can it achieve? Nat Clin Pract Neurol, 3(3), 152-161. Bergman, U., Ostergren, A., Gustafson, A. L., & Brittebo, B. (2002). Differential effects of olfactory toxicants on olfactory regeneration. Arch Toxicol, 76(2), 104-112. Borders, A. S., Getchell, M. L., Etscheidt, J. T., van Rooijen, N., Cohen, D. A., & Getchell, T. V. (2007a). Macrophage depletion in the murine olfactory epithelium leads to increased neuronal death and decreased neurogenesis. J Comp Neurol, 501(2), 206-218. Borders, A. S., Hersh, M. A., Getchell, M. L., van Rooijen, N., Cohen, D. A., Stromberg, A. J., et al. (2007b). Macrophagemediated neuroprotection and neurogenesis in the olfactory epithelium. Physiol Genomics, 31(3), 531-543. Boruch, A. V., Conners, J. J., Pipitone, M., Deadwyler, G., Storer, P. D., Devries, G. H., et al. (2001). Neurotrophic and migratory properties of an olfactory ensheathing cell line. Glia, 33(3), 225-229. Boyd, J. G., Lee, J., Skihar, V., Doucette, R., & Kawaja, M. D. (2004). Lacz-expressing olfactory ensheathing cells do not associate with myelinated axons after implantation into the compressed spinal cord. Proc Natl Acad Sci U S A, 101(7), 2162-2166. Bundesen, L. Q., Scheel, T. A., Bregman, B. S., & Kromer, L. F. (2003). Ephrin-b2 and ephb2 regulation of astrocytemeningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci, 23(21), 7789-7800. 192 M.J. Ruitenberg and J. Vukovic / Promoting central nervous system regeneration Bunge, M. B., & Pearse, D. D. (2003). Transplantation strategies to promote repair of the injured spinal cord. J Rehabil Res Dev, 40(4 Suppl 1), 55-62. Caggiano, M., Kauer, J. S., & Hunter, D. D. (1994). Globose basal cells are neuronal progenitors in the olfactory epithelium: A lineage analysis using a replication-incompetent retrovirus. Neuron, 13(2), 339-352. Carr, V. M., & Farbman, A. I. (1993). The dynamics of cell death in the olfactory epithelium. Exp Neurol, 124(2), 308-314. Chen, H., Kohno, K., & Gong, Q. (2005). Conditional ablation of mature olfactory sensory neurons mediated by diphtheria toxin receptor. J Neurocytol, 34(1-2), 37-47. Chen, J., Magavi, S. S., & Macklis, J. D. (2004). Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc Natl Acad Sci U S A, 101(46), 16357-16362. Christensen, M. D., Holbrook, E. H., Costanzo, R. M., & Schwob, J. E. (2001). Rhinotopy is disrupted during the re-innervation of the olfactory bulb that follows transection of the olfactory nerve. Chem Senses, 26(4), 359-369. Chuah, M. I., & Au, C. (1991). Olfactory schwann cells are derived from precursor cells in the olfactory epithelium. J Neurosci Res, 29(2), 172-180. Chuah, M. I., Choi-Lundberg, D., Weston, S., Vincent, A. J., Chung, R. S., Vickers, J. C., et al. (2004). Olfactory ensheathing cells promote collateral axonal branching in the injured adult rat spinal cord. Exp Neurol, 185(1), 15-25. Chung, R. S., Woodhouse, A., Fung, S., Dickson, T. C., West, A. K., Vickers, J. C., et al. (2004). Olfactory ensheathing cells promote neurite sprouting of injured axons in vitro by direct cellular contact and secretion of soluble factors. Cell Mol Life Sci, 61(10), 1238-1245. Costanzo, R. M. (2000). Rewiring the olfactory bulb: Changes in odor maps following recovery from nerve transection. Chem Senses, 25(2), 199-205. Costanzo, R. M., & Graziadei, P. P. (1983). A quantitative analysis of changes in the olfactory epithelium following bulbectomy in hamster. J Comp Neurol, 215(4), 370-381. Cowan, C. M., Thai, J., Krajewski, S., Reed, J. C., Nicholson, D. W., Kaufmann, S. H., et al. (2001). Caspases 3 and 9 send a pro-apoptotic signal from synapse to cell body in olfactory receptor neurons. J Neurosci, 21(18), 7099-7109. Cui, Q., Pollett, M. A., Symons, N. A., Plant, G. W., & Harvey, A. R. (2003). A new approach to CNS repair using chimeric peripheral nerve grafts. J Neurotrauma, 20, 17-31. Cummings, D. M., Emge, D. K., Small, S. L., & Margolis, F. L. (2000). Pattern of olfactory bulb innervation returns after recovery from reversible peripheral deafferentation. J Comp Neurol, 421(3), 362-373. David, S., & Aguayo, A. J. (1981). Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science, 214(4523), 931-933. Davies, S. J., Fitch, M. T., Memberg, S. P., Hall, A. K., Raisman, G., & Silver, J. (1997). Regeneration of adult axons in white matter tracts of the central nervous system. Nature, 390(6661), 680-683. Davies, S. J., Goucher, D. R., Doller, C., & Silver, J. (1999). Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci, 19(14), 5810-5822. De Winter, F., Oudega, M., Lankhorst, A. J., Hamers, F. P., Blits, B., Ruitenberg, M. J., et al. (2002). Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp Neurol, 175(1), 61-75. Deckner, M. L., Risling, M., & Frisen, J. (1997). Apoptotic death of olfactory sensory neurons in the adult rat. Exp Neurol, 143(1), 132-140. Deumens, R., Koopmans, G. C., Den Bakker, C. G., Maquet, V., Blacher, S., Honig, W. M., et al. (2004). Alignment of glial cells stimulates directional neurite growth of cns neurons in vitro. Neuroscience, 125(3), 591-604. Deumens, R., Koopmans, G. C., Honig, W. M., Hamers, F. P., Maquet, V., Jerome, R., et al. (2006a). Olfactory ensheathing cells, olfactory nerve fibroblasts and biomatrices to promote long-distance axon regrowth and functional recovery in the dorsally hemisected adult rat spinal cord. Exp Neurol, 200(1), 89-103. Deumens, R., Koopmans, G. C., Lemmens, M., Mollers, S., Honig, W. M., Steinbusch, H. W., et al. (2006b). Neurite outgrowth promoting effects of enriched and mixed oec/onf cultures. Neurosci Lett, 397(1-2), 20-24. Doucette, J. R., Kiernan, J. A., & Flumerfelt, B. A. (1983). The re-innervation of olfactory glomeruli following transection of primary olfactory axons in the central or peripheral nervous system. J Anat, 137 (Pt 1), 1-19. Doucette, R. (1991). Pns-cns transitional zone of the first cranial nerve. J Comp Neurol, 312(3), 451-466. Doucette, R. (1993). Glial progenitor cells of the nerve fiber layer of the olfactory bulb: Effect of astrocyte growth media. J Neurosci Res, 35(3), 274-287. Emsley, J. G., Mitchell, B. D., Kempermann, G., & Macklis, J. D. (2005). Adult neurogenesis and repair of the adult cns with neural progenitors, precursors, and stem cells. Prog Neurobiol, 75(5), 321-341. Fairless, R., Frame, M. C., & Barnett, S. C. (2005). N-cadherin differentially determines schwann cell and olfactory ensheathing cell adhesion and migration responses upon contact with astrocytes. Mol Cell Neurosci, 28(2), 253-263. Farbman, A. I., & Margolis, F. L. (1980). Olfactory marker protein during ontogeny: Immunohistochemical localization. Dev Biol, 74(1), 205-215. Fawcett, J. W., & Keynes, R. J. (1990). Peripheral nerve regeneration. Annu Rev Neurosci, 13, 43-60. Fernandes, K. J., Fan, D. P., Tsui, B. J., Cassar, S. L., & Tetzlaff, W. (1999). Influence of the axotomy to cell body distance in rat rubrospinal and spinal motoneurons: Differential regulation of gap-43, tubulins, and neurofilament-m. J Comp Neurol, 414(4), 495-510. Feron, F., Perry, C., Cochrane, J., Licina, P., Nowitzke, A., Urquhart, S., et al. (2005). Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain, 128(Pt 12), 2951-2960. Fouad, K., Schnell, L., Bunge, M. B., Schwab, M. E., Liebscher, T., & Pearse, D. D. (2005). Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci, 25(5), 1169-1178. M.J. Ruitenberg and J. Vukovic / Promoting central nervous system regeneration Franssen, E. H., de Bree, F. M., & Verhaagen, J. (2007). Olfactory ensheathing glia: Their contribution to primary olfactory nervous system regeneration and their regenerative potential following transplantation into the injured spinal cord. Brain Res Rev, 56(1), 236-258. Fry, F. J., & Cowan, W. M. (1972) A study of retrograde cell degeneration in the lateral mammillary nucleus of the cat, with special reference to the role of axonal branching in the preservation of the cell. J Comp Neurol, 144, 1-24. Giehl, K. M., & Tetzlaff, W. (1996). Bdnf and nt-3, but not ngf, prevent axotomy-induced death of rat corticospinal neurons in vivo. Eur J Neurosci, 8(6), 1167-1175. Goldshmit, Y., Galea, M. P., Wise, G., Bartlett, P. F., & Turnley, A. M. (2004). Axonal regeneration and lack of astrocytic gliosis in epha4-deficient mice. J Neurosci, 24(45), 10064-10073. Gong, Q., Bailey, M. S., Pixley, S. K., Ennis, M., Liu, W., & Shipley, M. T. (1994). Localization and regulation of low affinity nerve growth factor receptor expression in the rat olfactory system during development and regeneration. J Comp Neurol, 344(3), 336-348. 193 Kafitz, K. W., & Greer, C. A. (1999). Olfactory ensheathing cells promote neurite extension from embryonic olfactory receptor cells in vitro. Glia, 25(2), 99-110. Kawauchi, S., Beites, C. L., Crocker, C. E., Wu, H. H., Bonnin, A., Murray, R., et al. (2004). Molecular signals regulating proliferation of stem and progenitor cells in mouse olfactory epithelium. Dev Neurosci, 26(2-4), 166-180. Kempermann, G., Kuhn, H. G., & Gage, F. H. (1998). Experienceinduced neurogenesis in the senescent dentate gyrus. J Neurosci, 18(9), 3206-3212. Kobayashi, N. R., Fan, D. P., Giehl, K. M., Bedard, A. M., Wiegand, S. J., & Tetzlaff, W. (1997). Bdnf and nt-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate gap43 and talpha1-tubulin mrna expression, and promote axonal regeneration. J Neurosci, 17(24), 9583-9595. Kwon, B. K., Liu, J., Messerer, C., Kobayashi, N. R., McGraw, J., Oschipok, L., et al. (2002). Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury. Proc Natl Acad Sci U S A, 99(5), 3246-3251. Graziadei, P. P., Levine, R. R., & Monti Graziadei, G. A. (1979). Plasticity of connections of the olfactory sensory neuron: Regeneration into the forebrain following bulbectomy in the neonatal mouse. Neuroscience, 4(6), 713-727. Lakatos, A., Barnett, S. C., & Franklin, R. J. (2003). Olfactory ensheathing cells induce less host astrocyte response and chondroitin sulphate proteoglycan expression than schwann cells following transplantation into adult cns white matter. Exp Neurol, 184(1), 237-246. Hains, B. C., Black, J. A., & Waxman, S. G. (2003). Primary cortical motor neurons undergo apoptosis after axotomizing spinal cord injury. J Comp Neurol, 462(3), 328-341. Leaver, S. G., Harvey, A. R., & Plant, G. W. (2006). Adult olfactory ensheathing glia promote the long-distance growth of adult retinal ganglion cell neurites in vitro. Glia, 53(5), 467-476. Harding, J., Graziadei, P. P., Monti Graziadei, G. A., & Margolis, F. L. (1977). Denervation in the primary olfactory pathway of mice. Iv. Biochemical and morphological evidence for neuronal replacement following nerve section. Brain Res, 132(1), 11-28. Lee, V. M., & Pixley, S. K. (1994). Age and differentiation-related differences in neuron-specific tubulin immunostaining of olfactory sensory neurons. Brain Res Dev Brain Res, 83(2), 209-215. Harvey, A.R., Hu, Y., Leaver, S.G., Mellough, C.B., Park, K., Verhaagen, J., Plant, G.W., & Cui, Q. (2006). Gene therapy and transplantation in CNS repair: the visual system. Prog Retin Eye Res, 25, 449-489. Harvey, A. R., & Tan, M. M. L. (1992). Spontaneous regeneration of retinal ganglion cell axons in adult rats. NeuroReport, 3, 239-242. Hayat, S., Thomas, A., Afshar, F., Sonigra, R., & Wigley, C. B. (2003a). Manipulation of olfactory ensheathing cell signaling mechanisms: Effects on their support for neurite regrowth from adult cns neurons in coculture. Glia, 44(3), 232-241. Hayat, S., Wigley, C. B., & Robbins, J. (2003b). Intracellular calcium handling in rat olfactory ensheathing cells and its role in axonal regeneration. Mol Cell Neurosci, 22(2), 259-270. Heumann, R., Korsching, S., Bandtlow, C., & Thoenen, H. (1987). Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol, 104(6), 1623-1631. Hinds, J. W., Hinds, P. L., & McNelly, N. A. (1984). An autoradiographic study of the mouse olfactory epithelium: Evidence for long-lived receptors. Anat Rec, 210(2), 375-383. Leung, C. T., Coulombe, P. A., & Reed, R. R. (2007). Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci, 10(6), 720-726. Li, Y., Carlstedt, T., Berthold, C. H., & Raisman, G. (2004). Interaction of transplanted olfactory-ensheathing cells and host astrocytic processes provides a bridge for axons to regenerate across the dorsal root entry zone. Exp Neurol, 188(2), 300-308. Li, Y., Field, P. M., & Raisman, G. (1997). Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science, 277(5334), 2000-2002. Li, Y., Field, P. M., & Raisman, G. (1998). Regeneration of adult rat corticospinal axons induced by transplanted olfactory ensheathing cells. J Neurosci, 18(24), 10514-10524. Li, Y., Field, P. M., & Raisman, G. (2005a). Olfactory ensheathing cells and olfactory nerve fibroblasts maintain continuous open channels for regrowth of olfactory nerve fibres. Glia, 52(3), 245-251. Li, Y., Li, D., & Raisman, G. (2005b). Interaction of olfactory ensheathing cells with astrocytes may be the key to repair of tract injuries in the spinal cord: The ‘pathway hypothesis’. J Neurocytol, 34(3-5), 343-351. Jang, W., Youngentob, S. L., & Schwob, J. E. (2003). Globose basal cells are required for reconstitution of olfactory epithelium after methyl bromide lesion. J Comp Neurol, 460(1), 123-140. Li, Y., Yamamoto, M., Raisman, G., Choi, D., & Carlstedt, T. (2007). An experimental model of ventral root repair showing the beneficial effect of transplanting olfactory ensheathing cells. Neurosurgery, 60(4), 734-740; discussion 740-731. John, J. A., & Key, B. (2003). Axon mis-targeting in the olfactory bulb during regeneration of olfactory neuroepithelium. Chem Senses, 28(9), 773-779. Lipson, A. C., Widenfalk, J., Lindqvist, E., Ebendal, T., & Olson, L. (2003). Neurotrophic properties of olfactory ensheathing glia. Exp Neurol, 180(2), 167-171. 194 M.J. Ruitenberg and J. Vukovic / Promoting central nervous system regeneration Lu, P., Yang, H., Culbertson, M., Graham, L., Roskams, A. J., & Tuszynski, M. H. (2006). Olfactory ensheathing cells do not exhibit unique migratory or axonal growth-promoting properties after spinal cord injury. J Neurosci, 26(43), 11120-11130. Pastrana, E., Moreno-Flores, M. T., Avila, J., Wandosell, F., Minichiello, L., & Diaz-Nido, J. (2007). Bdnf production by olfactory ensheathing cells contributes to axonal regeneration of cultured adult cns neurons. Neurochem Int, 50(3), 491-498. Mackay-Sim, A., & Kittel, P. (1991a). Cell dynamics in the adult mouse olfactory epithelium: A quantitative autoradiographic study. J Neurosci, 11(4), 979-984. Pastrana, E., Moreno-Flores, M. T., Gurzov, E. N., Avila, J., Wandosell, F., & Diaz-Nido, J. (2006). Genes associated with adult axon regeneration promoted by olfactory ensheathing cells: A new role for matrix metalloproteinase 2. J Neurosci, 26(20), 5347-5359. Mackay-Sim, A., & Kittel, P. W. (1991b). On the life span of olfactory receptor neurons. Eur J Neurosci, 3(3), 209-215. Magavi, S. S., Leavitt, B. R., & Macklis, J. D. (2000). Induction of neurogenesis in the neocortex of adult mice. Nature, 405(6789), 951-955. Pearse, D. D., & Bunge, M. B. (2006). Designing cell- and genebased regeneration strategies to repair the injured spinal cord. J Neurotrauma, 23(3-4), 438-452. Margolis, F. L. (1972). A brain protein unique to the olfactory bulb. Proc Natl Acad Sci U S A, 69(5), 1221-1224. Pearse, D. D., Marcillo, A. E., Oudega, M., Lynch, M. P., Wood, P. M., & Bunge, M. B. (2004). Transplantation of Schwann cells and olfactory ensheathing glia after spinal cord injury: does pretreatment with methylprednisolone and interleukin-10 enhance recovery? J Neurotrauma, 21(9), 1223-1239. Mason, M. R., Lieberman, A. R., & Anderson, P. N. (2003). Corticospinal neurons up-regulate a range of growth-associated genes following intracortical, but not spinal, axotomy. Eur J Neurosci, 18(4), 789-802. Matulionis, D. H. (1975). Ultrastructural study of mouse olfactory epithelium following destruction by znso4 and its subsequent regeneration. Am J Anat, 142(1), 67-89. McKeon, R. J., Schreiber, R. C., Rudge, J. S., & Silver, J. (1991). Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci, 11(11), 3398-3411. Moon, L. D., Leasure, J. L., Gage, F. H., & Bunge, M. B. (2006). Motor enrichment sustains hindlimb movement recovered after spinal cord injury and glial transplantation. Restor Neurol Neurosci, 24(3), 147-161. Moreno-Flores, M. T., Lim, F., Martin-Bermejo, M. J., Diaz-Nido, J., Avila, J., & Wandosell, F. (2003). High level of amyloid precursor protein expression in neurite-promoting olfactory ensheathing glia (oeg) and oeg-derived cell lines. J Neurosci Res, 71(6), 871-881. Moulton, D. G. (1974). Dynamics of cell populations in the olfactory epithelium. Ann N Y Acad Sci, 237(0), 52-61. Myckatyn, T. M., Mackinnon, S. E., & McDonald, J. W. (2004). Stem cell transplantation and other novel techniques for promoting recovery from spinal cord injury. Transpl Immunol, 12(3-4), 343-358. Nandoe Tewarie, R. D., Hurtado, A., Levi, A. D., Grotenhuis, J. A., & Oudega, M. (2006). Bone marrow stromal cells for repair of the spinal cord: Towards clinical application. Cell Transplant, 15(7), 563-577. O’Toole, D. A., West, A. K., & Chuah, M. I. (2007). Effect of olfactory ensheathing cells on reactive astrocytes in vitro. Cell Mol Life Sci, 64(10), 1303-1309. Oudega, M. (2007). Schwann cell and olfactory ensheathing cell implantation for repair of the contused spinal cord. Acta Physiol (Oxf), 189(2), 181-189. Pasterkamp, R. J., Giger, R. J., Ruitenberg, M. J., Holtmaat, A. J., De Wit, J., De Winter, F., et al. (1999). Expression of the gene encoding the chemorepellent semaphorin iii is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal cns. Mol Cell Neurosci, 13(2), 143-166. Pearse, D. D., Sanchez, A. R., Pereira, F. C., Andrade, C. M., Puzis, R., Pressman, Y., et al. (2007). Transplantation of schwann cells and/or olfactory ensheathing glia into the contused spinal cord: Survival, migration, axon association, and functional recovery. Glia, 55(9), 976-1000. Pellitteri, R., Spatuzza, M., Russo, A., & Stanzani, S. (2007). Olfactory ensheathing cells exert a trophic effect on the hypothalamic neurons in vitro. Neurosci Lett, 417(1), 24-29. Plant, G. W., Bates, M. L., & Bunge, M. B. (2001). Inhibitory proteoglycan immunoreactivity is higher at the caudal than the rostral schwann cell graft-transected spinal cord interface. Mol Cell Neurosci, 17(3), 471-487. Plant, G. W., Christensen CL, Oudega M, Bunge MB. (2003). Delayed transplantation of olfactory ensheathing glia promotes sparing/regeneration of supraspinal axons in the contused adult rat spinal cord. J Neurotrauma, 20, 1-16. Plunet, W., Kwon, B. K., & Tetzlaff, W. (2002). Promoting axonal regeneration in the central nervous system by enhancing the cell body response to axotomy. J Neurosci Res, 68(1), 1-6. Raisman, G., & Li, Y. (2007). Repair of neural pathways by olfactory ensheathing cells. Nat Rev Neurosci, 8(4), 312-319. Ramer, L. M., Au, E., Richter, M. W., Liu, J., Tetzlaff, W., & Roskams, A. J. (2004a). Peripheral olfactory ensheathing cells reduce scar and cavity formation and promote regeneration after spinal cord injury. J Comp Neurol, 473(1), 1-15. Ramer, L. M., Richter, M. W., Roskams, A. J., Tetzlaff, W., & Ramer, M. S. (2004b). Peripherally-derived olfactory ensheathing cells do not promote primary afferent regeneration following dorsal root injury. Glia, 47(2), 189-206. Ramon-Cueto, A., Cordero, M. I., Santos-Benito, F. F., & Avila, J. (2000). Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron, 25(2), 425-435. Ramon-Cueto, A., & Nieto-Sampedro, M. (1994). Regeneration into the spinal cord of transected dorsal root axons is promoted by ensheathing glia transplants. Exp Neurol, 127(2), 232-244. Ramon-Cueto, A., Perez, J., & Nieto-Sampedro, M. (1993). In vitro enfolding of olfactory neurites by p75 ngf receptor positive ensheathing cells from adult rat olfactory bulb. Eur J Neurosci, 5(9), 1172-1180. M.J. Ruitenberg and J. Vukovic / Promoting central nervous system regeneration Ramon-Cueto, A., Plant, G. W., Avila, J., & Bunge, M. B. (1998). Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci, 18(10), 3803-3815. Ramon y Cajal, S. (1928, 1991). Degeneration and Regeneration of the Nervous System. (DeFelipe, J., Jones, E.G., May, R.M., eds.). NewYork: Oxford University Press. Reier, P. J. (2004). Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx, 1(4), 424-451. Reiter, E. R., DiNardo, L. J., & Costanzo, R. M. (2004). Effects of head injury on olfaction and taste. Otolaryngol Clin North Am, 37(6), 1167-1184. Richardson, P. M., McGuinness, U. M., & Aguayo, A. J. (1980). Axons from CNS neurons regenerate into PNS grafts. Nature, 284(5753), 264-265. Richter, M. W., Fletcher, P. A., Liu, J., Tetzlaff, W., & Roskams, A. J. (2005). Lamina propria and olfactory bulb ensheathing cells exhibit differential integration and migration and promote differential axon sprouting in the lesioned spinal cord. J Neurosci, 25(46), 10700-10711. Richter, M. W., & Roskams, A. J. (2007). Olfactory ensheathing cell transplantation following spinal cord injury: Hype or hope? Exp Neurol. 195 Schwob, J. E. (2002). Neural regeneration and the peripheral olfactory system. Anat Rec, 269(1), 33-49. Schwob, J. E., Youngentob, S. L., & Meiri, K. F. (1994). On the formation of neuromata in the primary olfactory projection. J Comp Neurol, 340(3), 361-380. Schwob, J. E., Youngentob, S. L., & Mezza, R. C. (1995). Reconstitution of the rat olfactory epithelium after methyl bromideinduced lesion. J Comp Neurol, 359(1), 15-37. Sonigra, R. J., Brighton, P. C., Jacoby, J., Hall, S., & Wigley, C. B. (1999). Adult rat olfactory nerve ensheathing cells are effective promoters of adult central nervous system neurite outgrowth in coculture. Glia, 25(3), 256-269. Stokols, S., & Tuszynski, M. H. (2006). Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials, 27(3), 443-451. Suzuki, Y., Schafer, J., & Farbman, A. I. (1995). Phagocytic cells in the rat olfactory epithelium after bulbectomy. Exp Neurol, 136(2), 225-233. Suzuki, Y., Takeda, M., & Farbman, A. I. (1996). Supporting cells as phagocytes in the olfactory epithelium after bulbectomy. J Comp Neurol, 376(4), 509-517. Riddell, J. S., Enriquez-Denton, M., Toft, A., Fairless, R., & Barnett, S. C. (2004). Olfactory ensheathing cell grafts have minimal influence on regeneration at the dorsal root entry zone following rhizotomy. Glia, 47(2), 150-167. Takami, T., Oudega, M., Bates, M. L., Wood, P. M., Kleitman, N., & Bunge, M. B. (2002). Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci, 22(15), 6670-6681. Rieger, A., Deitmer, J. W., & Lohr, C. (2007). Axon-glia communication evokes calcium signaling in olfactory ensheathing cells of the developing olfactory bulb. Glia, 55(4), 352-359. Tennent, R., & Chuah, M. I. (1996). Ultrastructural study of ensheathing cells in early development of olfactory axons. Brain Res Dev Brain Res, 95(1), 135-139. Ruitenberg, M. J., Blits, B., Dijkhuizen, P. A., te Beek, E. T., Bakker, A., van Heerikhuize, J. J., et al. (2004). Adeno-associated viral vector-mediated gene transfer of brain-derived neurotrophic factor reverses atrophy of rubrospinal neurons following both acute and chronic spinal cord injury. Neurobiol Dis, 15(2), 394-406. Tetzlaff, W., Alexander, S. W., Miller, F. D., & Bisby, M. A. (1991). Response of facial and rubrospinal neurons to axotomy: Changes in mrna expression for cytoskeletal proteins and gap-43. J Neurosci, 11(8), 2528-2544. Ruitenberg, M. J., Plant, G. W., Christensen, C. L., Blits, B., Niclou, S. P., Harvey, A. R., et al. (2002). Viral vector-mediated gene expression in olfactory ensheathing glia implants in the lesioned rat spinal cord. Gene Ther, 9(2), 135-146. Ruitenberg, M. J., Plant, G. W., Hamers, F. P., Wortel, J., Blits, B., Dijkhuizen, P. A., et al. (2003). Ex vivo adenoviral vectormediated neurotrophin gene transfer to olfactory ensheathing glia: Effects on rubrospinal tract regeneration, lesion size, and functional recovery after implantation in the injured rat spinal cord. J Neurosci, 23(18), 7045-7058. Tetzlaff, W., Kobayashi, N. R., Giehl, K. M., Tsui, B. J., Cassar, S. L., & Bedard, A. M. (1994). Response of rubrospinal and corticospinal neurons to injury and neurotrophins. Prog Brain Res, 103, 271-286. Tisay, K. T., Bartlett, P. F., & Key, B. (2000). Primary olfactory axons form ectopic glomeruli in mice lacking p75ntr. J Comp Neurol, 428(4), 656-670. Tisay, K. T., & Key, B. (1999). The extracellular matrix modulates olfactory neurite outgrowth on ensheathing cells. J Neurosci, 19(22), 9890-9899. Toft, A., Scott, D. T., Barnett, S. C., & Riddell, J. S. (2007). Electrophysiological evidence that olfactory cell transplants improve function after spinal cord injury. Brain, 130(Pt 4), 970-984. Santos-Silva, A., Fairless, R., Frame, M. C., Montague, P., Smith, G. M., Toft, A., et al. (2007). Fgf/heparin differentially regulates schwann cell and olfactory ensheathing cell interactions with astrocytes: A role in astrocytosis. J Neurosci, 27(27), 71547167. Turner, C. P., & Perez-Polo, J. R. (1993). Expression of p75ngfr in the olfactory system following peripheral deafferentation. Neuroreport, 4(8), 1023-1026. Sasaki, M., Hains, B. C., Lankford, K. L., Waxman, S. G., & Kocsis, J. D. (2006). Protection of corticospinal tract neurons after dorsal spinal cord transection and engraftment of olfactory ensheathing cells. Glia, 53(4), 352-359. Turner, C. P., & Perez-Polo, J. R. (1994). Changes in expression of the low affinity receptor for neurotrophins, p75ngfr, in the regenerating olfactory system. Int J Dev Neurosci, 12(8), 767773. Sasaki, M., Lankford, K. L., Zemedkun, M., & Kocsis, J. D. (2004). Identified olfactory ensheathing cells transplanted into the transected dorsal funiculus bridge the lesion and form myelin. J Neurosci, 24(39), 8485-8493. Ubink, R., Halasz, N., Zhang, X., Dagerlind, A., & Hokfelt, T. (1994). Neuropeptide tyrosine is expressed in ensheathing cells around the olfactory nerves in the rat olfactory bulb. Neuroscience, 60(3), 709-726. 196 M.J. Ruitenberg and J. Vukovic / Promoting central nervous system regeneration Ubink, R., & Hokfelt, T. (2000). Expression of neuropeptide y in olfactory ensheathing cells during prenatal development. J Comp Neurol, 423(1), 13-25. van den Pol, A. N., & Santarelli, J. G. (2003). Olfactory ensheathing cells: Time lapse imaging of cellular interactions, axonal support, rapid morphologic shifts, and mitosis. J Comp Neurol, 458(2), 175-194. Vavrek, R., Pearse, D. D., & Fouad, K. (2007). Neuronal populations capable of regeneration following a combined treatment in rats with spinal cord transection. J neurotrauma, 24(10), 16671673. Verhaagen, J., Oestreicher, A. B., Gispen, W. H., & Margolis, F. L. (1989). The expression of the growth associated protein b50/gap43 in the olfactory system of neonatal and adult rats. J Neurosci, 9(2), 683-691. Verhaagen, J., Oestreicher, A. B., Grillo, M., Khew-Goodall, Y. S., Gispen, W. H., & Margolis, F. L. (1990). Neuroplasticity in the olfactory system: Differential effects of central and peripheral lesions of the primary olfactory pathway on the expression of b-50/gap43 and the olfactory marker protein. J Neurosci Res, 26(1), 31-44. Vincent, A. J., Taylor, J. M., Choi-Lundberg, D. L., West, A. K., & Chuah, M. I. (2005a). Genetic expression profile of olfactory ensheathing cells is distinct from that of schwann cells and astrocytes. Glia, 51(2), 132-147. Vincent, A. J., West, A. K., & Chuah, M. I. (2005b). Morphological and functional plasticity of olfactory ensheathing cells. J Neurocytol, 34(1-2), 65-80. Vukovic, J., Plant, G.W., Ruitenberg, M.J., Harvey, A.R. (2007). Influence of adult Schwann cells and olfactory ensheathing glia on axon-target cell interactions in the CNS: a comparative analysis using a retinotectal co-graft model. Neuron Glia Biol, 3, 105-117. Wang, F., Nemes, A., Mendelsohn, M., & Axel, R. (1998). Odorant receptors govern the formation of a precise topographic map. Cell, 93(1), 47-60. Wehrle, R., Camand, E., Chedotal, A., Sotelo, C., & Dusart, I. (2005). Expression of netrin-1, slit-1 and slit-3 but not of slit-2 after cerebellar and spinal cord lesions. Eur J Neurosci, 22(9), 2134-2144. Wewetzer, K., Grothe, C., & Claus, P. (2001). In vitro expression and regulation of ciliary neurotrophic factor and its alpha receptor subunit in neonatal rat olfactory ensheathing cells. Neurosci Lett, 306(3), 165-168. Wewetzer, K., Kern, N., Ebel, C., Radtke, C., & Brandes, G. (2005). Phagocytosis of o4+ axonal fragments in vitro by p75- neonatal rat olfactory ensheathing cells. Glia, 49(4), 577-587. Whitman, M. C., & Greer, C. A. (2007). Adult-generated neurons exhibit diverse developmental fates. Dev Neurobiol, 67(8), 1079-1093. Williams, S. K., Franklin, R. J., & Barnett, S. C. (2004a). Response of olfactory ensheathing cells to the degeneration and regeneration of the peripheral olfactory system and the involvement of the neuregulins. J Comp Neurol, 470(1), 50-62. Williams, S. K., Gilbey, T., & Barnett, S. C. (2004b). Immunohistochemical studies of the cellular changes in the peripheral olfactory system after zinc sulfate nasal irrigation. Neurochem Res, 29(5), 891-901. Windus, L. C., Claxton, C., Allen, C. L., Key, B., & St John, J. A. (2007). Motile membrane protrusions regulate cell-cell adhesion and migration of olfactory ensheathing glia. Glia, 55(16), 1708-1719. Woodhall, E., West, A. K., & Chuah, M. I. (2001). Cultured olfactory ensheathing cells express nerve growth factor, brain-derived neurotrophic factor, glia cell line-derived neurotrophic factor and their receptors. Brain Res Mol Brain Res, 88(1-2), 203213. Xu, X. M., Chen, A., Guénard, V., Kleitman, N., Bunge, M. B. (1997). Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J Neurocytol, 26(1), 1-16. Zhang, Y., Tohyama, K., Winterbottom, J. K., Haque, N. S., Schachner, M., Lieberman, A. R., et al. (2001). Correlation between putative inhibitory molecules at the dorsal root entry zone and failure of dorsal root axonal regeneration. Mol Cell Neurosci, 17(3), 444-459. Zou, D. J., Feinstein, P., Rivers, A. L., Mathews, G. A., Kim, A., Greer, C. A., et al. (2004). Postnatal refinement of peripheral olfactory projections. Science, 304(5679), 1976-1979.