* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
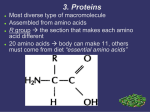
Download • What are enzymes? They`re special type of protein that accelerates
Survey
Document related concepts
Restriction enzyme wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Genetic code wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Catalytic triad wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Proteolysis wikipedia , lookup
Metalloprotein wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Biosynthesis wikipedia , lookup
Transcript
Slide 1 • • • • Slide 2 • What are enzymes? They`re special type of protein that accelerates the chemical reaction without being consumed during it (they undergo changes during the reaction, they interfere with it but they get out at the end of the reaction exactly the way they entered, going back to their original shape) Generally, enzymes are globular proteins with only one exception which is Ribozymes(special type of RNA that can speed up chemical reactions, consisting of a special sequence of nucleotides making up the nucleic acid) other enzymes are all globular proteins. Enzymes are the most efficient catalysts known, other catalysts can speed up the reaction between 102 and 104 multiplied by the original speed, but protein catalysts which are enzymes can increase the rate of the reaction up to 1020 , so they`re the best catalysts known almost all enzymes specially the ones found in the human body are finely regulated by a lot of processes Energy of the chemical reaction is expressed by the equation: ∆G=∆H-T∆S The sign of ΔG depends on the signs of the changes in enthalpy (ΔH) and entropy (ΔS), as well as on the absolute temperature (T, in Kelvin). ΔG changes from positive to negative (or vice versa) where T = ΔH/ΔS. • • • • • • • • • • For a chemical reaction to occur there must be a difference in energy between reactants and products When the energy of reactants > the energy of products , the reaction is spontaneous , favorable, exergonic and exothermic When the energy of reactants < the energy of products , the reaction is non-‐spontaneous, non-‐favorable, endergonic and endothermic When ∆G is negative the reaction is exergonic (if the energy is in the form of heat it`s exothermic) and it gives you energy. But when it`s positive the reaction is endergonic and it needs energy to occur(if the energy is in the form of heat it`s endothermic) Why do chemical reactions take place? to keep homeostasis and get stability in life Why isn`t everything reacting with everything? Because of activation energy needed to start the reaction and that energy isn`t always available When we give the reactants the activation energy needed we transform them into a higher energy state known as the transitional state, which can be defined as a particular configuration along the reaction coordinate corresponding to the highest energy along it ∆G0 of a reaction is the difference of the reactants and products when the reaction is taking place under standard conditions which are room temperature, pressure of 1 () and 1 molar concentration with preservation of the condition of concentration Any building up reaction needs energy to occur on the other side any breaking up reaction releases energy If a reaction is endergonic the reverse of it is exergonic and vice versa, with the same value of energy difference but with different signs Slide 3 Slide 4 Slide 5 Slide 6 Slide 7 Why are enzymes important? No chemical reaction in the human body can naturally occur without the help of enzymes, so they`re extremely important, also outside the human body enzymes can be very useful, example of tea in the slides and corn syrup which is a substance a lot like honey produced with the help of enzymes, it gives sweet flavor. Also, sucrose the disaccharide is broken down with the help of enzymes( in the production of chocolate when there`s soft chocolate inside a hard one outside, what makes the inside soft is the presence of yeast enzymes which break up sucrose). Finally amoxicillin the most widely used antibiotic is also produced with the help of enzymes Classification of enzymes : • Oxidoreductases : they catalyzes the oxidation and reduction reactions in which transfer of electrons occur in the form of H,O,O2 etc. what exactly is the thing which loses or gains electrons? It`s a coenzyme having the ability to gain or lose, one example is the heme in hemoglobin and myglobin, it involves the binding of oxygen to the ion changing from +2 to +3 and vice versa. They require the presence of two reactants at least ( example in the slides) • Transferases: they help transform a group from one molecule to another, they require the presence of two reactants leading to the production of two products example in the slides • Hydrolazes: add water to macromolecules so it can split and one example is the split of proteins into amino acids adding water to split the peptide bond • Isomerazes: they change the configuration of a molecule without changing its shape, it requires or acts on one molecule or one substrate • Lysases: addition or removal of one molecule to a chemical structure, without splitting it like adding to a double bond for example( two materials ending up as one) • Ligases: connects two materials together so we have two materials and one product, they are usually involved in the building processes so they need energy which they get usually from being coupled to reactions that gives energy most likely from ATP degradation • Classification of enzymes according to structure: They can be classified as if we were talking about proteins into simple: sequence of amino acids by itself that can form tertiary or quaternary structure and then be active unlike conjugated: sequence of amino acids that require a non-‐protein part to be fully active(may be also called complex enzymes), the material that binds to the enzyme is what`s called the coenzyme • Coenzyme: is anon protein part that binds to the enzyme either covalently or not covalently and it`s needed for the enzyme to be active • Conjugated enzymes if the coenzyme is bound to it, it`s called Holoenzyme, if not it`s called Apoenzyme • Enzyme-‐substrate binding: reaction occurs inside a pocket of the enzyme structure that`s called the active site, what`s the active site? It`s a pocket lined by amino acids not necessarily a sequence of amino acids it may be amino acid #100 beside the one of #307 for example, the important part is that they are arranged in a special shape making the groove that can help with the acceleration of the reaction of course that groove is lined with amino acids, the nature of these amino acids determine the activity of the enzyme • • Slide 8 Slide 9 Slide 10 Inside the active site the reactants or substrates bind to the enzyme chemically, and there the enzyme transform them into the transitional state which has higher energy than both the reactants and products Enzymes speed up reaction but they don`t affect any of it`s characteristics such as ∆G for example, the only provide an alternative pathway for the reaction to proceed through decreasing the activation energy and making it easier to get to the transitional state, it affects the activation energy only, making it lower, they don`t change the favorability of the reaction • Enzymes have specificity, to stereoisomers for example, some enzymes work on the D-‐ isomers and others work on the L-‐ isomers Range of enzymes` specificity varies, some are highly specific(working on only one material) and others have lower specificity or even hypodrant specificity but no enzyme in the universe is not specific at all because the function is affected with the enzymes` specifity • If the reaction is spontaneous that doesn`t mean it`s fast, it can be spontaneous and slow! Rate of the reaction is determined by the ability of the enzyme to lower the activation energy • Catalase is an enzyme that can transform the hydrogen peroxide into oxygen and water, it`s present in almost all cells, you can put cells in a test tube and after a while you`ll notice bubbles coming out of the tube , an indication of the production of oxygen due to the breaking down of the peroxide with the help of the catalaze provided by the degeneration of cells , this enzyme is highly specific and the rate of the reaction is very high it reaches 108 which is the highest rate reached ever in nature There are two theories proposed throughout history to explain how enzymes work: • The first: lock and key: the binding site is ready and modified to accept a specific substrate , and it was proven wrong! Proteins are not rigid in nature, they`re constantly moving and they work dynamically. • The second: induced fit model: the binding site has a specific shape but when bound to the substrate it changes shape to a more closed shape, the relaxed fit of the enzyme allow it to accept more than one substrate and it goes better with the dynamic nature of proteins, once the enzyme is bound to the substrate it changes shape and catalyzes the reaction Effects the binding site can exert on the substrate : • Proximity effect: the active site of the enzyme brings the substrate close to it in order for the reaction to happen • Orientation effect: the active site has specific amino acids lining it, they may be hydrophobic, hydrophilic, negatively or positively charged , etc.. the substrate come close to it and binds, the negative with the positive, the hydrophobic together and so on, so the substrate come close to the enzyme in the orientation specified by the amino acid lining, this effect is called the orientation effect • Catalytic effect: amino acids present in the enzyme have a catalytic effect , the amino acids make bonds with the substrate , for example: the serine has a hydroxyl group OH that binds with histidine amino acid, • • when the substrate binds to the enzyme this bond breaks and each amino acid bind with a part of the substrate, which leads to a higher energy of the substrate moving it to the transitional state Energy effect: it`s very close to the catalytic effect , it`s the effect the enzyme exerts on the substrate by lowering the energy barrier and it does so by inducing strain in the substrate bonds, and that of course leads to instability of the molecule which all aids in the breaking of the bonds present and forming new ones in the products ﻳﯾﻌﻁطﻳﯾﻛﻡم ﺍاﻟﻌﺎﻓﻳﯾﺔJ Best Regards Rawan Jaras