* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 14 Inside the Atom Notes
Survey
Document related concepts
Transcript
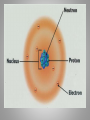
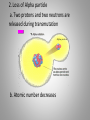
Inside the Atom Section 1 and 2 Section 1 Models of the Atom Models of the Atom A.Greek philosophers devised a theory of atoms, or tiny particles (around 500 B.C.). B. John Dalton combined the idea of elements with the Greek theory of the atom (early 1800s). 1. Matter is made up of atoms. 2. Atoms cannot be divided into smaller pieces. 3. All atoms of an element are exactly alike 4. Different elements are made of different atoms. 5. Dalton’s theory was tested by William Crookes and his cathode-ray tube experiment. C. J.J. Thomson discovered negatively charged particles, electrons, which are a part of every atom (1897). 1. Thomson revised Dalton’s model to include a sphere with a positive charge and negatively charged electrons spread evenly within the positive charge. 2. The negatively charged electrons and the positive charge in the sphere neutralized each other. D. Ernest Rutherford tested Thomson’s model, which was found to be an inaccurate model of the atom. E. An atomic model with a nucleus was developed (1911). 1. The positively charged proton is located in a very small space at the center of an atom. 2. Most of an atom is empty space occupied by nearly massless electrons. 3. Electrically neutral particles, neutrons, are also located in the nucleus. 4. The number of electrons equals the number of protons in an atom. F. The electron cloud model explains the unpredictable wave behavior of electrons, which could be anywhere in the area surrounding the nucleus. Discussion Question What particles make up an atom, and what electrical charge do they have? Electrons: negatively charged Protons: positively charged Neutrons: electrically neutral Section 2 The Nucleus A. Atomic number – number of protons in the nucleus of an atom. 1. Isotopes of an atom have the same number of protons but different numbers of neutrons (pg. 415). 2. Mass number is the number of neutrons plus the number of protons. 3. Average atomic mass – the average mass of the mixture of an element’s isotopes 4. The strong nuclear force holds tightly packed protons together in a nucleus. What happens when something decays? B. Radioactive decay occurs when an atom releases nuclear particles and energy. 1. When a proton is released, one element changes into another, a process called transmutation. It’s Morphing Time!!! 2. Loss of Alpha particle a. Two protons and two neutrons are released during transmutation b. Atomic number decreases 3. Loss of Beta particle a. A high-energy electron from the nucleus is released with energy when an unstable neutron splits into an electron and proton. b. Atomic number increases by one. C. Half-life of a radioactive isotope is the amount of time it takes for half the sample to decay. 1. Half-lives range in length from fractions of second to billions of years. 2. Carbon-14 dating is used to determine the age of artifacts and fossils. 3. Radioactive waste must be disposed of carefully to avoid harming people and the environment. D. Synthetic elements are made in labs by smashing atomic particles into a target element. 1. Radioactive isotopes from artificial transmutation are called tracer elements and can used for medical purposes. 2. Tracer elements are also used to study the environmental impact of pesticides and fertilizers and to locate water resources. Discussion Question What are two ways radioactive decay can occur? Through the release of alpha particles (two protons and two electrons). And through the release of a beta particle (highenergy electron from a split neutron.


























![Properties of matter student notes[1]](http://s1.studyres.com/store/data/009076956_1-3293fc3fecf578fd34e3f0f2700d471f-150x150.png)






