* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download electric charge
Anti-gravity wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Electromagnetism wikipedia , lookup
Speed of gravity wikipedia , lookup
Elementary particle wikipedia , lookup
Maxwell's equations wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Fundamental interaction wikipedia , lookup
Magnetic monopole wikipedia , lookup
Work (physics) wikipedia , lookup
Lorentz force wikipedia , lookup
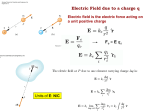
PHYSICS ELECTROSTASTICS ELECTROSTATICS ELECTRIC CHARGE Charge is the property associated with matter due to which is produces and experiences electrical and magnetic effects. The study of electrical effects of charge at rest is called electrostatics. The strength of particle’s electric interaction with objects around it depends on its electric charge, which can be either positive or negative. An object with equal amounts of two kinds of charge is electrically neutral, whereas one with an imbalance is electrically charged. In the table given below, if a body in the first column is rubbed against a body in the second column, the body in first column will acquire positive charge, while that in the second column will acquire negative charge. TABLE I Sl. No 1. 2. 3. 4. 5. First Column Glass Rod Flannes or cat skin Woollen cloth Woollen cloth Woollen cloth Second Column Silk Rod Ebonite rod Amber Rubber shoes Plastics objects Electric Charge: Electric charge can be written as ne where n is a positive or negative integer and e is a constant of nature called the elementary charge (approximately 1.60 x 10-19C). Electric charge is conserved, the (algebraic) net charge of any isolated system cannot be changed. Regarding charge following points are worth nothing: (a) Like charges repel each other and unlike charges attract each other. (b) Charge is a scalar and can be of two types; positive or negative. (c) Charge is quantized, i.e., the charge on anybody will be some integral multiple of e, i.e., Q - ± ne. where n- 1, 2, 3…………… (d) Charge on anybody can never be (1/3e), 1.5e etc. The electrostatic unit of charge is stat-coulomb and electromagnetic unit is abcoulomb in CGS system. But in SI system the unit of charge is coulomb, I coulomb =1/10ab-coulomb = 3x109 stat-coulomb. NOTE: Recently, it has been discovered that elementary particles such as proton or neutron are composed of quarks having charge (±1/3)e and (±2/3)e. However, as quarks do not exist in Free State, the quantum of charge is still e. Example-1: How many electrons are there in one coulomb of negative charge? Sol: The negative charge is due to presence of excess electrons, since they carry negative charge. Because an electron has a charge whose magnitude is e = 1.6x10 -19 C, the number of electrons N=q/e=1.0/1.6x10-19 N=6.25x1018 (5) Unit and dimensional formula S.I. unit of charge is Ampere x sec = coulomb ©, smaller S.I. units are mC, µC. C.G.S. unit of charge is Stat coulomb or e.s.u. Electromagnetic unit of charge is ab coulomb 1C=3x109 stat coulomb = 1/10 ab coulomb. Dimensional formula [Q]=[AT] (6) Charge is Transferable: It can be transferred from one body to another Associated with mass: Charge cannot exist without mass but reverse is not true. Conserved: It can neither be created nor be destroyed. Invariant: Independent of velocity of charged particle. → → (7) Electric charge produced electric field (E), magnetic field (B) and electromagnetic radiations. → → → + v = 0 + v = constant + v ≠ constant → E only → → E and B →→ E, B and radiates energy (8) Point charge: A finite size body may behave like a point charge if it produces an inverse square electric field. For example an isolated charged sphere behave like a point charge at very large distance as well as very small distance close to it’s surface. (9) Charge on a conductor: Charge given to a conductor always resides on it’s outer surface. This is way a solid and hollow conducting sphere of same outer radius will hold maximum equal charge. If surface is uniform the charge distributes uniformly on the surface and for irregular surface the distribution of charge, i.e., Charge density is not uniform. It is maximum where the radius of curvature is minimum and vice versa. i.e., (1/R). This is why charge leaks from sharp points. (10) Charge Distribution: It may be of two types (i) Discrete distribution of charge: A System consisting of ultimate individual charges (ii) Continuous distribution of charge: An amount of charge distribute uniformly or non-uniformly on a body. It is of following three types (a) Line charge distribution: Charge on a line e.g. charged straight wire, circular charged ring etc. Charge = Linear charge density Length S.I. unit is C/M Diamension is [L-1TA] (b) Surface charge distribution: Charge distributed on surface e.g. plane sheet of charge, conducting sphere, conducting cylinder of =Charge = Surface charge density Area S.I. Unit is C/m2 (c) Volume charge density: Charge distributes through out the volume of the body e.g. charge on a dielectric sphere = charge= Volume charge density Volume S.I. Unit is C/m3 Method of Charging: A body can be charged by following methods. (1) By Friction: By rubbing two bodies together, both positive and negative charges in equal amounts appear simultaneously due to transfer of electrons from one body to the other. (i) When a glass rod is rubbed with silk, the rod becomes positively charged while the silk becomes negatively charged. The decrease in the mass of glass rod is equal to the total mass of electrons lost by it. (ii) Ebonite on rubbing with wool becomes negatively charged making the wool positively charged. (iii) Clouds also get charged by friction. (iv) A comb moving through dry hair gets electrically charged. It starts attracting small bits of paper. (2) By electrostatic induction: If a charged body is brought near an uncharged body, one side of neutral body (closer to charged body) becomes oppositely charged while the other side becomes similarly charged. Induced charge can be lesser or equal to inducing charge (but never greater) and its maximum value is given by Q’=-Q[1-1/K] Where Q is the inducing charge and K is the dielectric constant of the material of the uncharged body. It is also known as specific inductive capacity (SIC) of the medium, or relative permittivity Er of the medium (relative means with respect to free space) Different dielectric constants Medium Vaccum Air Paraffin Wax Rubber Transformer oil Glass K 1 1.0003 2.1 3 4.5 5-10 Medium Mica Silicon Germanium Glycerin Water Metal K 6 12 16 50 80 (3) Charging by conduction: Take two conductors, one charged and other uncharged. Bring the conductors in contact with each other. The charge (whether –ve or +ve) under its own repulsion will spread over both the conductors. Thus the conductors will be charged with the same sign. This is called as charging by conduction (through contact). 1 Coulomb = 3 X 109 esu of charge = emu of charge (1 Faraday = 96500 coulomb, 1 Amp Hr = 3600 coulombs) The esu of charge is also called Static coloumb (stat. coul.) or frankline (Fr). = emu of charge = 3X 1010 = C esu of charge Frankline (i.e. esu of charge) is the smallest unit of charge, while faraday is largest. [Remember – Faraday is unit of capacity] Properties of charge – 1. Like charges repel and opposite charges attract. Ex. – A (+)ve charge sphere will attract – Sol. – (i) (-) ve charged (ii) Neutral 2. Charge is a scalar and can be of two types only viz positive and negative. This is because it adds algebraically and represents excess or deficiency of electrons. 3. Charge is transferable – If a charged body is put in contact with an uncharged body, uncharged body becomes charged due to transfer of electrons from one body to the other. If the charged body is positive and it will withdraw some electrons from uncharged body and if negative will transfer some of its excess electrons to the uncharged body. The process of charge transfer is called ‘Conduction’ and in conduction – 1. The charged body loses some of its charge (which is equal to the charge gained by uncharged body). 2. The charges on both the books are similar if initially one is charged and other uncharged. 3. The charge gained by uncharged body is always lesser than initial charge present on charged body, i.e. whole of the charge cannot be transferred by conduction from one body to the other. Actually, the flow of charge stops when both acquire same potential. Exception:Ex: Can ever the whole charge of a body be transferred to the other? If yes how and if not why? Ans: Yes, if the charged body is enclosed by a conducting body and connected to it, the whole charge will be transferred to the conducting body, as charge resides on the outer surface of a conductor. 4. Charge is invariant – This means that charge like phase is independent of frame of reference, i.e. charge on a body does not charge whatever be its speed. While charge density or mass of a body depends on its speed and increases with increase in speed. 5. Charge is always associated with Mass I.e. charge cannot exist without mass though mass can exist without charge, so: a. The particles such as photon or neutrino which have no (rest) mass can never have a charge (as charge cannot exist without mass). b. As charge cannot exist without mass, the presence of charge is a convincing proof of existence of mass. c. In charging, the mass of a body charges. 6. Charge is conserved – In isolated system, total charge does not charge with time, though individual charges may charge i.e. charge can neither be created nor destroyed. It therefore, follows that simultaneously equal quantities of positive and negative charge can appear or disappear. This is what actually happens in pair production and anniweatron. Conservation of charge is also found to hold good in all types of reactions either chemical, nuclear or decay. In pair production and anniweatron neither mass nor energy is conserved separately but (mass + energy) is conserved. In pair production, presence of nucleus is a must to conserve momentum. In absence of nucleus, both energy and momentum will not be conserved simultaneously and the process cannot take place. 7. Accelerated charge radiates energy: Electromagnetic theory has established that a charged particle at rest produces only electric field in the space surrounding it. However, if the charged particle is in unaccelerated motion it produces both electric and magnetic fields but doesnot radiate energy. And if the motion of charged particle is accelerated it not only produces electric and magnetic fields but also radiates energy in the space surrounding the charge in the form of electromagnetic waves. V E V = Constant E & B But no radiation V ≠ Constant E, B and radiates energy 8. Similar charges repel each other while dissimilar attract. The True test of electrification is repulsion and not attraction as attraction may also take place between a charged and an uncharged body and also between two similarly charged bodies. Quest: Can two similarly charged bodies ever attract each other? Ans: Yes, when the charge on one body (Q) is much greater than that on the other (q) and they are close enough to each other so that force of attraction between (Q) and induced charge on the other exceeds the force of repulsion between (Q) and (q). If the charges are point, no induction will take place and hence, two similar point charges can never attract each other. 9. Charge resides on the outer surface of a conductor because like charge repel and try to get as far as possible from one another and stay at the farthest distance from each other which is outer surface of the conductor. This is why a solid and a hollow conductor sphere of same outer radius soap bubble expand on charging. 10. In case of conducting body no doubt charge resides on its outer surface, the distribution of charge, i.e. charge density is not uniform. It is maximum where the radius of curvature is minimum and vice-versa, i.e. σ α (1/R). This is why charge leaks from sharp points. Proof: a. As conductor is an equipotential surface, i.e. Vs = constant and incase of spherical conductor 1 q 4rf 0 R with q = 4rR 2 v 1 4rR 2 v = So, o s = Cons tan t 4rf 0 R 1 i.e. vR = Cons tan t or va R os = b. Lighting rods are made up of conductors with one of their ends earthed while the other sharp and protects a building rom lighting either by neutralizing or conducting charge of the cloud to the ground. 11. A body can be charged by friction, induction or conduction. In friction when two bodies are rubbed together, electrons are transferred from one body to the other. As a result of this one body becomes positively charged while the other negatively charged. E.g. when a glass rod is rubbed with silk, the rod becomes positively charged while the silk negatively. However, ebonite on rubbing with wool becomes negatively charged making the wool positively charged. Clouds also become charged by friction. In charging by friction in accordance with conservation of charge, both positive and negative charges in equal amounts appear simultaneously due to transfer of electrons from one body to the other. In case of induction it is worth noting that – 1. Inducing body neither gains nor loses charge. 2. The nature of induced charge is always opposite to that of inducing charge is always opposite to that of inducing charge. 3. Induced charge can be lesser or equal to inducing charge (but never greater) and its maximum value is given by – q’ = -q(1 – 1/K) Where q is the inducing charge and K is the dielectric constant of the material of the uncharged body. 4. For metals in electrostatics, k = 3, So, q’ = -q i.e. in metals induced charge is equal and opposite to inducing charge. 5. Induction takes place only in bodies (either conducting or non-conducting) and not in particles. 12. If a charged body is brought near a neutral body, the charged body will attract opposite charge and repel similar charge present in the neutral body. As a result of this one side of the neutral body becomes positive while the other negative this process is called “Electrostatic Induction”. 13. Charge can be detected and measured with the help of gold leaf electroscope, electrometer, voltammeter or ballistic – galvanometer. In case of gold leaf electroscope – a. If a charged body is brought near uncharged electroscope, charge on the disc of electroscope will be opposite to that of body while leaves similar to that of body and leaves while diverse. b. If an uncharged electroscope is touched by a charged body, disc and leaves both acquire charge similar to that of body and leaves will diverse. c. If electroscope is charged by induction, disc and leaves both will acquire charge opposite to that of inducting body and leaves will diverse. In fig © electroscope is charged by induction using a positive charged body. d. If a charged body is brought near a charged electroscope, the leaves will further diverse if the charge on the body is similar to that on the electroscope and will usually converge if opposite. This is how we determine the nature of charge. If the induction effect is strong enough leaves offer converging may again diverse. Ex: What is the difference between ‘charging by induction and charging by conduction’? 1. In induction the two bodies are ‘close to each other’, while in conduction touch each other. 2. In induction charge on inducing body remains uncharged while in conduction charge on charging body charges. 3. In induction induced charge is always opposite in nature to the ‘inducing charge’ while in conduction the charge on the two bodies is always of same nature. 4. In ‘induction’ induced charge can be equal in magnitude to inducing charge but in conduction ‘charge transferred’ is usually lesser than initial charge present. COULOMB’s LAW – Coulomb found that force between two point charges at rest – 1. Varies directly as the magnitude of each charge, i.e. Fα q1 X q2 2. Varies inversely as the square of distance between them, i.e. Fα 1/r 2 3. Depends on the nature of medium between the charges. 4. Is always along the line joining the charges. 5. Is attractive if charges are unlike and repulsive if like. Fα q1 X q2 α 1/r2 Fair = 1 q1 q2 4rf 0 r 2 Fmed = q1 q2 1 4rf 0 K r 2 1 = Km 2 9X109 2 (SI Unit) 4rf0 c K = Constant = Characterizes the medium between the charges and is called dielectric constant, specific inductive capacity (S.I.C) or relative permittivity and for vaccum, free space or air its value is taken to be 1. F12 = q1 q2 r| 4rf0 Kr3 12 q1 q2 = 1 r 12 4rf r3 f = f0 K f K = = f r (relative permittivity or f0 dielectric cons tan t) r q1 K q2 F12 = Force on q1 due to q2. S r12 = Unit vector directed to q1 from q2 F12 ! f0 = 8.85 X 10-12 F/m The equilibrium of a charged particle under the action of Colombian forces along can never be stable. This statement is known as Earnshaw’s Theorem. Ex: A copper atom consists of copper nucleus surrounded by 29 electrons. The atomic weight of copper is 63.5g/mol. Let us now take two pieces of copper each weighing 10g. Let us transfer one electron from one piece to another for every 1000 atoms in a piece. What will be the Coulomb force between the two pieces after the transfer of electrons if they are 2.10cm apart? 23 Sol.: No of atoms in 10gm of copper = 6X 10 X 10 = 9.45 X 1022 63.5 Total electron transferred = 1 X 9.45 X 1022 = 9.45 X 1019 1000 q = ne = 9.45 X 1019 X 1.6 X 10-19 = 15.12c Treating each piece of copper as point charge, electric force between them from coulomb’s law when they are 10 cm apart F= Ex. Sol. 9 X 109 X (15.2) 2 = 2.08 X 1014 3 (10 X 10-2) 2 (a) Two similar point charges q1 and q2 are placed at a distance r apart in air. If a dielectric slab of thickness t and dielectric const. K is put between the charges, calculate the coulomb force of repulsion. (b) If the thickness of the slab covers half the distance between the charges, the coulombs force repulsive is reduced 4 : 9. Calculate the in the ratio dielectric constant of the slab. (a) 1 q1q2 1 q1q2 2 40 r ' 40 Kr2 r' r K If there exists a slab of thickness t and dielectric constant K, the effective air separation between the charges will be: F= (b) q1q2 1 40 r t t K 2 F 4 F0 9 q1q2 r r 2 1 40 4 1 = 9 40 r K 2 q1q2 r2 2 K=4 PRINCIPLE OF SUPERPOSITION OF COULOMB'S LAW: The resultant force on a test charge is a vector sum of forces due to individual charges. Fres F1 F2 F3 F4 Equilibrium of Charge Particle: If the net force acting on the charge particle is zero that we say that, the charge particle is in equilibrium. Equilibrium of a charge Case (Q1, Q2) when Q1 and Q2 both are of similar nature Let |Q1| < |Q2| For q to be in equilibrium, Fq = 0, KqQ1 KQ2q 2 2 x r x Q1 Q2 x rx x Q1 Q1 Q2 r nearer to charge of smaller magnitude. Case 2: Q1 and Q2 when Q1 and Q2 are of opposite nature. KQ1q x2 F1 = KQ2q F2 = r x 2 KQ1q KQ2q 2 2 x r x Q1 x2 Q2 r x 2 Q1 Q2 x rx x Q2 r Q1 x Q1 x r Q1 x= r Q1 Q1 Q2 From charge of smaller magnitude. Ex. Two point charges +q and +4q are placed at a distance and apart. Find the magnitude, sign and location of a third charge which makes the system in equilibrium. Force on charge q due to 4q (repulsive) (in the direction of BA). In order to make A in equilibrium, a negative charge (let q 1) be placed between A and B at a distance x from A. For the equilibrium of A 1 q 4q 1 q q1 40 l2 40 x2 q4x2 = l2 q1 Considering the equilibrium of C, 1 q1 4q 1 q q1 2 40 l x 40 x2 4x2 = (l – x)2 2x = + (l – x) x= l 3 l 4q 3 q1 l2 Ex. 2 4q 9 A pith ball of mass 9 × 10–5 kg carries a charge of 5 µc. What must be the magnitude and sign of the charge on a pith ball B held 2 cm directly above the pith ball A, such that the pith ball A remain's stationary? FAB = m1g = 1 q1q2 40 AB2 92 = 7.84 µC. Ex. Three identical spheres each having a charge q and radius R, are kept in such away that each touches the other two. Find the magnitude of the electric force on any sphere due to other two. FAB = 1 q2 BA 40 2R 2 FAC 1 q2 CA 40 2R 2 FA 3 FAB 1 3 9 = 40 4 R Ex. 2 Two equally charged identical metal spheres A and B repel each other with a force 2 × 10–5 N. Another identical uncharged sphere C is touched to B and then placed at the mid point between A and B. What is the net electric force on C? [R 1981] K q2 F= r2 = 2 × 10–5 N when sphere C touches B, the charge on B, q will distribute equally on B and C as spheres are identical conductors. For conductors in contact V1 = V2 q1 q2 r1 r2 r1 r2 q1 q2 Also q 1 + q2 = q q1 q2 q 2 So sphere C will experience a force FCA = q Kq 2 r 2 2 2F along AB due to charge on A. FCB = q q K 2 2 r 2 2 F along BA due to charge on B. So the net force on C due to charges on A and B FC = FCA – FCB = 2F – F = F along AB Ex. Three identical spheres each having a charge q and radius R, are kept in such a way that each touches the other two. Find the magnitude of the electric force on any sphere due to other two. Sol. As for external points a charged sphere behaves as if the whole of its charge where concentrated at its centre. Force on A due to B FAB = = 1 qq 40 2R 2 1 q2 42 4R 2 along BA Force on A due to C FAC = 1 qq 40 2R 2 1 q2 = 40 4R 2 along CA FAB = FAC = F FA = = F2 F2 2FF cos 60 3F 1 3 q FA = 40 4 R Ex. 2 Five point charges, each of value +q are placed on five vertices of a regular hexagon of side LM. What is the magnitude of the force on a point charge of value –q coulomb placed at the centre of a hexagon? If there had been a sixth charge +q at the remaining vertex of hexagon force due to all the six charges on –q at O will be zero (as the forces due to individual charges will balance each other), i.e., FR 0 Now if f is the force due to sixth charge and F due to remaining five charges, F F 0 i.e., F f or f = F = 1 qq 40 L2 F=f 1 q = 40 L Ex. 2 An α-particle passes rapidly, through the exact centre of a hydrogen molecule moving on a line perpendicular to the inter-nuclear axis. The distance -particle experience the maximum force and what is it? Sol. FR = 2F cos Ө along BA 1 2e2 F 40 x2 a2 x cos Ө = x 2 1 2 2 a with a = b 2 FR = 2 1 2e2 40 x2 a2 x e2x i.e., FR = 2 3 2 2 0 x a For FR to be max, dFR 0 dx x= a 2 2 x 1 2 2 a b Ans. 2 2 = 8e2 Fmax. = 3 3 0b2 Ex. A point charge q is situated at a distance d from one end of a thin non conducting rod of length L having a charge Q (uniformly distributed along its length). Find the magnitude of electric force between the two. dF = 1 qdQ 40 x2 dQ = Q dx L dF = 1 qQ dx 40 Lx2 1 qQ F= 40 L d L d dx x2 d L 1 qQ 1 = 40 L x d = 1 qQ 1 1 40 L d d L F= Ex. 1 qQ 40 d d L Two identical charged spheres are suspended by strings of equal length the strings make an angle of 30° with each other. When suspended in a liquid of density 0.8 gm/cc, the angle remains the same. What is the dielectric constant of the liquid? (Density of the material of sphere is 1.6 gm/cc). T cos Ө = mg T sin Ө = F …(2) ….(1) tan Ө = F mg ….(3) When the balls are suspended in a liquid of density σ and dielectric constant K, 1 times, i.e., K the electric force will become F while K F' = weight mg' = mg – th = mg – vσ g mg' = mg 1 V= m tan Ө' = = F' mg' F Kmg 1 ' K= Ex. 1.6 2 1.6 0.8 (a) Two similar helium filled spherical balloons tied to a 5 gm weight with strings and each carrying an electric charge q float in equilibrium as shown in fig. Find the magnitude of q in eqn assuming that the charge on each balloon acts as it were concentrated at its centre. (b) Find the volume of each balloon. Neglect the weight of the unfilled balloons and assume that the density of air = 0.00129 gm/cc and density of helium inside the balloon = 0.0002 gm/cc). Equilibrium of weight ….(1) Equilibrium of a balloon F = T sin Ө Th – mg = T cos Ө i.e. Vσ g – Vƿ g = T cos Ө …(3) F= mg tan 2 and Vg F= q= mg …(4) 2 qq in eqn. units x2 x2 and V = mg tan 2 m 2 = 1665 eqn of charge V= 5 2 0.00129 0.0002 5 105 = 218 = 2294 cc TRANSLATORY EQUILIBRIUM When several forces act on a body simultaneously in such a way that the resultant force on the body is zero, i.e., F0 With F Fi The body is said to be in translatory equilibrium. 1. As if a vector is zero all its components must vanish, i.e. in equilibrium as– F Fi 0 Fx 0, Fy 0 and Fz 0 So in equilibrium forces along x-axis must balance each other and same is true for other directions. 2. As for a body F0 means ma 0 or dV 0 dt V const or zero i.e., if a body is in translatory equilibrium it will be either at rest or in uniform motion. If it is at rest, the equilibrium is called static, otherwise dynamic. 3. If the forces are conservative, then as for conservative force dV F dr and for equilibrium (F = 0) So F = i.e., dV 0 dr dV 0 dr i.e., in conservative fields at equilibrium potential energy is optimum, i.e., in equilibrium potential energy is maximum or minimum or constant. 4. Dynamic equilibrium types: Types of dynamic equilibrium. (a) Stable equilibrium: If on slight displacement from equilibrium position a body has tendency to regain its original position, it is said to be in stable equilibrium. In case of stable equilibrium potential energy is d2V minimum 2 ve and so centre of gravity is lowest. dr (b) Unstable equilibrium: If on slight displacement from equilibrium position body moves in the direction of displacement, the equilibrium is said to be unstable. In this situation potential energy of the body is d2V maximum 2 negative and so centre of gravity is dr highest. Examples– (c) Neutral equilibrium: If on slight displacement from equilibrium position a body has no tendency to come back to original position or to move in the direction of displacement, it is said to be in neutral equilibrium. In this situation potential energy of the body is constant d2V 0 dr2 and so centre of gravity remains at constant height. 5. In case of stable equilibrium lesser the potential energy or lower the centre of gravity, i.e., greater the base area more stable is the equilibrium. 6. If we plot graphs between F U and , at equilibrium F will be zero r r while U will be optimum (max or min or constant). If U = min i.e., d2U dr 2 = (+) ve, equilibrium is stable. U = max d2U i.e., dr 2 = negative, equilibrium is unstable. and U = constt. d2U i.e., = 0, dr 2 Equilibrium is neutral. ELECTRIC FIELD AND POTENTIAL * The space surrounding an electric charge q in which another charge q0 experiences a (electrostatic) force of attraction, or repulsion, is called the electric field of the charge q. * q ԑ Source charge Point charge a group of point charges continuous distribution of charges q0 ԑ test charge must be vanishingly small so that it does not modify the Electric field of the source charge. Intensity (or strength) of Electric field– E F q0 The intensity of electric field at a point in an electric field is the ratio of the force acting on the test-charge placed at that point to the magnitude of the test-charge. If the intensity of electric field E at a point in an electric field be known, then we can determine the force F acting on a charge q placed at that point by the following eqn. F qE E MLT 3A 1 gl gl gl 2 2 1 mv2 2 v 2gl v l = 2gl l = 2g l 2g Ans. l Example-6: An inclined plane making an angle 30° with the horizontal is placed in a kg and charge 0.01 C is allowed to slide down from rest from a height of 1 m. If the coefficient of friction is 0.2, find the time it will take the particle to reach the bottom. Sol. The different forces on the particle are shown in figure. From, N = mg cos 30° + qԑ cos 60° Friction f = µN = µ mg cos 30° + µ ԑ cos 60° Now the total force F acting along the inclined plane is F = mg sin 30° – µN – qԑ cos 30° or F = mg sin 30° – mg cos 30° – µqԑ cos 60° – qԑ cos 30° Thus acceleration is or a = F m = g sin 30° – µg cos 30° q q cos 60 cos 30 m m or a = 9.8 × 0.5 – 0.2 × 9.8 3 0.2 0.01 100 0.01 100 3 0.5 1 1 2 2 Now, distance travelled in time t is s= 0 1 2 at 2 or t = 2 2 a [As s = 1 2] sin 30 or = 4 2.237 = 1.345 sec. Example-7: In space horizontal Electric field E mg exist as shown in figure and a q mass m attached at the end of a light rod. If mass m is released from the position shown in figure find the angular velocity of the rod when it passes through the bottom most position Sol. (A) g l (B) 2g l (C) 3g l (D) 5g l According to work energy theorem: w = ∆T WE + Wg = 1 mv2 0 ….(1) 2 WE = qE l sinӨ , Wg = mg (l – l cos Ө) – l cos Ө) = 1 mv2 from eqn. (1) 2 mg l sin Ө + mg l – mg l cos Ө = 1 mv2 2 mg QE q Electric Lines of Force: Faraday gave a new approach for representation of electric field in the form of electric lines of force. Electric lines of force are graphical representation of electric field. “An electric line of force is an imaginary line or curve drawn through a region of space so that its tangent at any point is in the direction of the electric field vector at that point.” This model of electric field has the following characteristics: (i) Electric lines of force are originated from positive charge and terminal into negative charge. (ii) The number of electric lines of force originates from a point charge q is q/ԑ0. Electric lines of force may be fraction. (iii) The number of lines per unit area that pass through a surface perpendicular to the electric field lines is proportional to the strength of field in that region. (iv) No electric lines of force cross each other. If two electric lines of force cross each other, it means electric field has two directions at the point of cross. This is not physically possible. (v) Electric lines of force for two equal positive point charges are said to have rotational symmetry about the axis joining the charges. (vi) Electric lines of force for point positive charge and a nearby negative point charge that are equal in magnitude are said to have rotational symmetry about an axis passing through both charges in the plane of the page. (vii) Electric dipole Electric lines of force due to infinitely large sheet of positive charge is normal to the sheet. (viii) No electrostatic lines of force are present inside a conductor. Also electric lines of force are perpendicular to the surface of conductor. For example if a conducting sphere is placed in a region where uniform electric field is present, then induced charges are developed on the sphere. (ix) If a charged particle is released from rest in region where only uniform electric field is present, then charged particle move along an electric line of force. But if charged particle has initial velocity, then the charged particle may or may not follow the electric lines of force. (x) Electric lines of force inside the parallel plate capacitor is uniform. It shows that field inside the parallel plate capacitor is uniform. But at the edge of plates, electric lines of force are curved. It shows electric lines of force at the edge of plates is non-uniform This is known as fringing effect. If the size of plates are infinitely large, then fringing effect can be neglected. (xi) If a metallic plate is introduced between plates of a charged capacitor, then electric lines of force can be discontinuous. (xii) If a dielectric plate is introduced between plates of a charged capacitor, then number of lines of forces in dielectric is lesser than that in case of vacuum space. (xiii) Electrostatics electric lines of force can never be closed loops, as a line can never start and end on the same charge. Also if a line of force is a closed curve, work done round a closed path will not be zero and electric field will not remain conservative. (xiv) Lines of force have tendency to contract longitudinally like a stretched elastic string producing attraction between opposite charges and repel each other laterally resulting in, repulsion between similar charges and edge-effect (curving of lines of force near the edges of a charged conductor). ELECTRIC POTENTIAL AND ELECTRIC POTENTIAL DIFFERENCE: Electric Potential: "Electric potential at any point in a electric field is equal to the ratio of the work done in bringing a test charge from infinity to that point, to the value of test charge." Suppose, W be the work required in bringing a test charge q 0 from infinity to a point b against the repulsive force F acting on it, then potential at the point b is Vb Wb q0 Since, W and q0 both are scalar quantities; the potential is also a scalar quantity. Electric Potential Difference: The potential difference between two points in an electric field is equal to the ratio of work done in moving a test charge from one point to the other, to the value of test charge. Suppose W work be done in bringing a small test charge q 0 from the point a to a point b against the repulsive force acting on it, then potential difference between the points is Vb Va Wab q0 Obviously, potential difference is also a scalar quantity. IMPORTANT FEATURES 1. Electric potential due to a point charge q: From the definition of potential, V U q0 1 qq0 . 40 r = q0 or V = 1 q . 40 r Here, r is the distance from the point charge q to be point at which the potential is evaluated. If q is positive, the potential that it produces is positive at all points; if q is negative, it produces a potential that is negative everywhere. In either case, V is equal to zero at r = 3 . 2. Electric potential due to a system of charges: Just as the electric field due to a collection of point charges is the vector sum of the fields produced by each charge, the electric potential due to a collection of point charges is the scalar sum of the potentials due to each charge. V 3. 1 40 qi r i i In the equation V 1 40 qi r , if i the whole charge is at equal i distance r0 from the point where V is to be evaluated, then we can write, V= 1 qnet . 40 r0 Where qnet is the algebraic sum of all the charges of which the system is made. Example-11: In a regular polygon of n sides, each corner is at a distance r from the centre. Identical charges are placed at (n – 1) corners. At the centre, the intensity is E and the potential is V. The ratio V has magnitude. E (A) (C) Sol. E= rn n 1 r (B) r(n – 1) (D) r n 1 n q 4 0 r2 and v n 1 q 4 0 r n 1 q 4 0 r v q E 4 0 r2 = (n – r) TABLE : Electric Potential of Various Systems S.No. 1. 2. First Column Isolated charge A ring of charge Second Column V q 40r E q 40 2 R x2 x 20 3. A disc of charge E 4. Infinite sheet of charge Not defined 5. Infinitely long line of charge Not defined 6. Finite line of charge V q R2 x2 sec tan ln 40 sec tan 7. Charged spherical shell (a) Inside 0 < r < R V (b) Outside r > R V 8. Solid sphere of charge (a) q 40r Inside 0 < r < R E (b) q 40R R 2 6 0 r2 3 R 2 Outside r > R V q 40r