* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Δk/k
Aharonov–Bohm effect wikipedia , lookup
Coherent states wikipedia , lookup
Renormalization group wikipedia , lookup
Schrödinger equation wikipedia , lookup
Dirac equation wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Casimir effect wikipedia , lookup
Canonical quantization wikipedia , lookup
Perturbation theory (quantum mechanics) wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Scalar field theory wikipedia , lookup
Rotational–vibrational spectroscopy wikipedia , lookup
Hydrogen atom wikipedia , lookup
Rotational spectroscopy wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Atomic theory wikipedia , lookup
Tight binding wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
2nd lecture:
Summary 1st lecture: Classical coupled vibrations
mx1 k1 x1 k ' ( x2 x1 )
mx2 k 2 x2 k ' ( x1 x2 )
x
k k' k'
.
mx Kx with x 1 , K 1
x2
k ' k2 k '
2
EIGENVALUES OF COUPLED OSCILLATORS
1.75
1.5
m
2
1.25
2k'
1
e
Characteristic equation det(K − eI) gives eigenvalues: mω2 e k k ' k ' 2 k 2 .
Y e k k ' , X k 2 form hyperbola Y 2 X 2 k ' 2 w.r.t. k (k k ' ) / 2 .
0.75
With ω ½(ω ω ) , ω ½(ω ω ) , solution for k = k' is
x1 (t ) x10 cos ω cos ω , x2 (t ) x10 sin ω sin ω .
0.5
0.25
4
General procedure:
q R T x with orthogonal R, leads to mq Dq , w. normal modes q.
Diagonal matrix D = RTKR has eigenvalues ei on its diagonal.
2
symmetric oscillator:
1
1
, sin 2 φ
1
1
2
amplitudes x1, x2
.
The solutions xi form vector space {xi } , with scalar product (xi·xj).
Various nomenclatures for mixing of orthogonal states,
2-dim: x1 q cos φ q sin φ
n-dim: | xi j | q j q j | xi
| xi j | q j q j | xi
x1 q (q x1 ) q (q x1 )
xi j cij q j
| x1 | q q | x1 | q q | x1
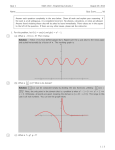
Generalization: n = 5 coupled oscillators: m i2 {3.732, 3., 2., 1. ,0.268}
x1(t)
1
0
x2(t)
-1
0
20
40
60
80
100
time t
STATE MIXING
1
cos2φ
0.8
sin2 and cos2
cos φ sin φ
,: cos 2 φ
R
sin
φ
cos
φ
10% ASYMM . COUPLED OSCILL ., Dk k ' = 2
2
2 dim: matrix of mixing coefficients, with asymmetry parameter k / k ' :
1
2
4
asymmetric oscillator:
Because of KR RD : The rows ri of the transformation matrix
are the eigenvectors of K: Kri ei ri with respect to the basis q.
Back-transformation then gives the solution x (t ) Rq(t ) .
2
2
k k'
3
1
2
0
0.6
0.4
0.2
sin2φ
4
2.1
2
0
2
k k'
4
2.
2.1
Two-state quantum systems: some basic experiments
The ammonia molecule NH3
3-dim structure:
a) the double well potential
In the NH3 molecule, the nitrogen atom can take two possible positions,
with respect to the mirror plane formed by the hydrogen-atoms:
N
N
z
| 1
or
| 2
The nitrogen atom sees a double-well potential V(z) along the axis z.
V(z)
V(z)
z
| 1
z
| 2
In its ground state, the molecule, the nitrogen atom is either in the one well,
which we call state | 1 , or in the other, which we call state | 2 .
2.2
b) coupled states
Harmonic oscillator:
Classically, the nitrogen atom must stay forever in its potential well | 1 or | 2 .
Quantum mechanically, the nitrogen atom can go from one state to the other,
because its wavefunctions | 1 or | 2 penetrate the classically forbidden regions.
We know this from the case of the harmonic oscillator. In the diagram, three different
quantities are plotted: the potential V(r), the total energies En, and the wavefcts. ψ(r).
Hence, the nitrogen atoms may tunnel through the potential wall from | 1 to | 2 and back.
Even if we do not know the exact shape of the potential V(z), we still can predict what will happen.
When there is no tunneling across the potential wall, then the states | 1 and | 2
are states of definite energy E0, that is | 1 and | 2 are also energy eigenstates,
i | 1 H | 1 E 0 | 1
and the Schrödinger equation reads
t
with solution | 1 | 1 0 exp( iE 0 t / ) for the nitrogen-atom on position 1.
1. When the molecule is in state | 1 , the probability to find it there is and remains
NH3-molecule:
V(z)
ψ(z)
E0
1 | 1 1 | 1 1 | 1 1 : because exp( iE 0 t / ) exp( iE 0 t / ) 1,
2
| 1
| 2
like in the classical case, and the same for the molecule in state | 2 .
2. When the two states | 1 , | 2 are coupled to each other by tunneling of the nitrogen atom,
then the state of the system in general is a superposition of | 1 and | 2 :
| ψ c1 | 1 c2 | 2 (which can, alternatively, be written | ψ | 1 1 | ψ | 2 2 | ψ ).
c
We therefore can define the state vector ψ 1 with respect to the basis | 1 , | 2 ,
c2
1
0
for instance: ψ ψ1 for the system in state | 1 , or ψ ψ 2 for the system in state | 2 .
0
1
2.3
c) matrix mechanics
In this case the Schrödinger equation in matrix representation reads
A c1
c E
.
iψ Hψ , with the energy matrix H, that is i 1 0
c2 A E0 c2
Due to the mirror symmetry of the problem, both diagonal elements E0 must be the same.
The off-diagonal elements in general are the complex conjugate of each other,
i.e. the matrix H is Hermitean, : H = H† = HT*, which ensures that its eigenvalues are real, Ei* = Ei.
In our 2-dim case with E1 = E2, A must be real, A* = A, and H is symmetric: H = HT.
E0 is the total energy of the molecule when it is definitely in one of the two states | 1 or | 2 ,
and A /τ is the transition amplitude for tunneling from | 1 to | 2 ,
where τ is the mean dwell time in | 1 or | 2 , i.e. 1/τ is the tunneling probability.
Pedestrian approach:
The Schrödinger equation iψ Hψ then is given by the two coupled differential equations
ic1 E0 c1 Ac2 ,
ic2 E0 c2 Ac1 .
Again, the two equations can be decoupled by taking their sum and their difference:
i(c1 c2 ) ( E0 A)(c1 c2 ) ,
i(c1 c2 ) ( E0 A)(c1 c2 ) .
c (c1 c 2 ) / 2 , c (c1 c 2 ) / 2 ,
Setting
this leads to the decoupled equations
ic ( E0 A)c ,
ic ( E0 A)c ,
with solutions c (t ) c (0) exp( i( E0 A)t /
c (t ) c (0) exp( i( E0 A)t / .
and
2.4
d) energy splitting
For the initial condition c1 (0) 1 , c2 (0) 0 , back transformation then gives
c1 (t ) (c (t) c (t )) / 2 12 exp( iE0t / )[exp( iAt / ) exp( iAt / )] , c12
that is c1 exp( iE 0 t / ) cos( At / ) .
In an energy eigenstate of the molecule, the probability
to find the nitrogen atom in state | 1 oscillates back and forth like:
c1
c2
2
cos 2 ( At / )
2
1 c1
2
t
c22
sin 2 ( At / ) , with frequency ω 2A / .
t
Again we can also derive the eigenvalues of H from
the secular equation det( H EI ) 0 , with unit matrix I, in the 2-dim case:
E0 E
A
A
E0 E
( E0 E ) 2 A 2 0 , or E0 E A ,
that is there are the two energy eigenvalues E E0 A and E E0 A
in agreement with the above.
If H does not change with time, the common phase factor exp( iE 0 t / )
in the time dependent Schrödinger equation cancels out,
and the stationary solution can be obtained from the
time independent Schrödinger equation Hψ = Eψ.
Of course, all this is an idealization,
the real picture is more like this:
1/λ:
barrier height: 2072 cm−1→
splitting: 36 cm−1→
0.8 cm−1→
E+
E−
2A
E0
Exited electronic state,
with molecular vibrational states,
Electronic ground state, with
molecular vibrational states υ = 0, 1, 2
and tunnel splitting (+ rotational)
2.5
e) general approach
The energy matrix H, being Hermitean, can be diagonalized: U†HU = E
with a unitary matrix U, that is UU† = I = unit matrix
(U† = UT* is the complex conjugate of UT).
Example: in the 2-dim case with real H, U is also real,
i.e. U is the same as for the symmetric coupled oscillator of chapter 1:
cos φ sin φ
1 1 1
, cf. page 1.5.
U
2 1 1
sin φ cos φ
Because of HU UE , the rows ri of the transformation matrix U then
are the energy eigenstates of H: Hui Ei ui , with respect to the basis | 1 , | 2 .
In the bra-ket nomenclature, the matrix diagonalization process U†HU = E
reads for the matrix elements of the Hamiltonian:
ψ|H | ψ ' i ψ|E i Ei|H|E i Ei|ψ ' i Ei ψ1|E i Ei|ψ 2 ,
where use has been made of the closure relation
|E E | 1 ,
i
i
i
where the |E i are the energy eigenstates,
and the |ψ are the | 1 , | 2 , ... base states.
2.6
|δ−| = 3|δ+|
z
f) The NH3 molecule in a static electric field
r
p
NH3 molecule has two 'dangling' electrons on the nitrogen atom,
with local excess charge -q on the nitrogen atom and +q/3 on the hydrogen atoms.
Hence, the molecule has an electric dipole moment p = q r.
Let the quantization axis z be directed along the symmetry axis of the molecule.
Then the molecule axis is directed only parallel or anti-parallel to z
(like every effective spin ½-system).
NB: The quantization axis z be directed anywhere,
but equations are simplest when z is directed along a symmetry axis.
~
In an external electric field E , directed along z,
~
~
the electric energy of the dipole is U p E p z E z ,
~ ~
that is the molecular energies of states | 1 , | 2 are, for p p z , E E z :
~
~
E E0 pE and E E0 pE ,
~
E 0 pE
A
H
and the energy matrix is
~ .
E 0 pE
A
Like in the case of the asymmetric oscillator, p.1.4, we then obtain
N
~
field E
N
z
~
from the secular equation the energy eigenvalues E E 0 A 2 p 2 E 2
2
~ 2
~
E E0 pE
1.
which, as function of E , lie on the two hyperbolas
A A
2.7
| 2
g) state mixing and anticrossing
1.75
Like in the case of the asymmetric coupled oscillator,
the matrix U that diagonalizes the Hamiltonian H contains the mixing coefficients
cos φ sin φ
, with
U
sin φ cos φ
1
1
, sin 2 φ
1
2
1
1
2
1.25
1
2A
0.75
| E
(| 1 | 2 ) / 2
0.5
| 1
0.25
| 2
4
2
~
, with pE / A .
~
In the energy eigenstate E+ of the molecule, when the field E along z is increased,
the increasing function P1 = cos 2 φ gives the probability that
the molecule is pulled into state | 1 ;
while the decreasing function P2 = sin 2 φ gives the probability that
the molecule remains in state | 2 .
(At field zero ξ = 0, the lower state is the symmetric one: | E (| 1 | 2 ) / 2 ,
because for vanishing barrier it must go over into the ground state of the
harmonic oscillator, which is also symmetric.)
0
2
4
ξ = pE A
for level E+:
| 2
1
STATE MIXING BY STARK EFFECT
sin 2 φ
| 1
P2
0.8
sin2 and cos2
cos 2 φ
2
| 1
| E
(| 1 | 2 ) / 2
1.5
E
The energy level diagram shows the typical level repulsion or anticrossing
of the energy levels of a coupled quantum mechanical system:
When there is state mixing, the levels repel each other.
1
2
ENERGIES OF NH3 MOLECULE IN E FIELD
2
0.6
P1
0.4
0.2
cos 2 φ
4
2
0
pE
A
ξ=
2
4
NB: All this is deduced without precise knowledge of V(z) or ψ(z).
N−
−
N−
~
field E
z
| 1
| 2
2.8
ENERGIES OF NH3 MOLECULE IN E FIELD
2
| 2
h) The nature of the Electric Dipole Moment (EDM)
1.75
| E
(| 1 | 2 ) / 2
1.5
The shifting of atomic energy due to an external electric field is called the Stark effect.
For comparison, for free atoms:
quadratic Stark eff., when they have an induced EDM,
linear Stark effect, when they have a permanent EDM.
1.25
1
E
In our molecular double well potential:
in low field we have a quadratic Stark effect,
in strong field we have a linear Stark effect.
| 1
0.75
| E
(| 1 | 2 ) / 2
0.5
| 1
0.25
| 2
4
2
0
2
4
ξ = pE A
Problem: within the Standard Model, a permanent EDM violates time reversal invariance.
peff
ξ
1 ξ 2
for level E+:
EFFECTIVE ELECTRIC DIPOLE MOMENT
1
0.5
peff
~ ~
~
For an EDM p p z in the electric field E E z , the energy is E pE ,
E
~
The effective EDM is defined as the slope of the function E (E ) : peff ~ .
E
2
2 ~2
With E E E0 A p E this is
~
p2E
2
2 ~2
p eff ~ A p E
~ ,
E
A2 p 2 E 2
0
peff = 0
~
p p , for not too large fields, with pE / A :
.
That is, in zero field, the NH3 molecule has no EDM:
The molecule only has an induced EDM.
The EDM in nonzero field comes about only by the fact that
the molecule is a coherent mixture of two states | 1 , | 2 , and
that the mixing becomes asymmetric when the field is applied.
0.5
1
4
2
0
ξ = pE A
2
4
Only for times shorter than the dwell time τ /A a nonzero mean EDM occurs, but for such short time
the splitting 2A of the energy levels is blurred by the energy-time uncertainty relation, so the effects of this short-time EDM are not visible.
2.9