* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Compound vs Element chart
Electron configuration wikipedia , lookup
Stoichiometry wikipedia , lookup
Electronegativity wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Safety data sheet wikipedia , lookup
Isotopic labeling wikipedia , lookup
List of phenyltropanes wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Chemical bond wikipedia , lookup
Alkaline earth metal wikipedia , lookup
Boron group wikipedia , lookup
Periodic table wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Organic chemistry wikipedia , lookup
Drug discovery wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Homoaromaticity wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Chemical element wikipedia , lookup
History of chemistry wikipedia , lookup
History of molecular theory wikipedia , lookup
Atomic theory wikipedia , lookup
Extended periodic table wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
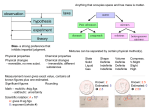
Name______________________Date____________ Elements,Compounds&Mixtures Part1:Readthefollowinginformationonelements,compoundsandmixtures. CompoundversusElementcomparisonchart Compound Element Meaning Acompoundcontainsatomsofdifferentelements chemicallycombinedtogetherinafixedratio. Anelementisapurechemicalsubstance madeofsametypeofatom. Distinguishing Feature Compoundscontaindifferentelementsinafixed ratioarrangedinadefinedmannerthrough chemicalbonds. Elementsaredistinguishedbytheiratomic number(numberofprotonsintheir nucleus). Abilityto Breakdown Acompoundcanbeseparatedintosimpler substancesbychemicalmethods/reactions. Elementscannotbebrokendowninto simplersubstancesbychemicalreactions. Types Thelistofcompoundsisendless. Thereareabout117elementsthathavebeen observed.Canbeclassifiedasmetal,nonmetalormetalloid. Representation Acompoundisrepresentedusingaformula. Anelementisrepresentedusingsymbols. Examples Water(H2O),Sodiumchloride(NaCl),Sodium Iron,copper,silver,gold,nickeletc. bicarbonate(NaHCO3)etc. Note that a compound: • consists of atoms of two or more different elements bound together, • can be broken down into a simpler type of matter (elements) by chemical means (but not by physical means), • has properties that are different from its component elements, and • always contains the same ratio of its component atoms. Note that an element: • consists of only one kind of atom, • cannot be broken down into a simpler type of matter by either physical or chemical means, and • can exist as either atoms (e.g. argon) or molecules (e.g., nitrogen). A molecule consists of two or more atoms of the same element, or different elements, that are chemically bound together. *Note that the two nitrogen atoms which comprise a nitrogen molecule move as a unit. Note that a mixture: • consists of two or more different elements and/or compounds physically intermingled, • can be separated into its components by physical means, and • often retains many of the properties of its components. Part2:FillintheBlanks Elements: • Apuresubstancecontainingonlyonekindof____________. • Anelementisalwaysuniformallthewaythrough(homogeneous). • Anelement_____________beseparatedintosimplermaterials(exceptduringnuclearreactions). • Over100existingelementsarelistedandclassifiedonthe____________________. Compounds: • Apuresubstancecontainingtwoormorekindsof_______________. • Theatomsare_________________combinedinsomeway.Oftentimes(butnotalways)theycome togethertoformgroupsofatomscalledmolecules. • Acompoundisalwayshomogeneous(uniform). • Compounds___________________beseparatedbyphysicalmeans.Separatingacompoundrequiresa chemicalreaction. • Thepropertiesofacompoundareusuallydifferentthanthepropertiesoftheelementsitcontains. Mixtures: • Twoormore________________or_________________NOTchemicallycombined. • Noreactionbetweensubstances. • Mixturescanbeuniform(called________________________)andareknownassolutions. • Mixturescanalsobenon-uniform(called________________________). • Mixturescanbeseparatedintotheircomponentsbychemicalorphysicalmeans. • Thepropertiesofamixturearesimilartothepropertiesofitscomponents. Part3:Classifyeachofthefollowingaselements(E),compounds(C)orMixtures(M).WritetheletterX ifitisnoneofthese. ___Diamond (C) ___Sugar(C6H12O6) ___Milk ___Iron(Fe) ___Air ___SulfuricAcid(H2SO4) ___Gasoline ___Electricity ___Krypton(K) ___Bismuth(Bi) ___Uranium(U) ___Popcorn ___Water(H2O) ___Alcohol(CH3OH) ___PailofGarbage ___Adog ___Ammonia(NH3) ___Salt(NaCl) ___Energy ___Gold(Au) ___Wood ___Bronze ___Ink ___Pizza ___DryIce(CO2) ___BakingSoda(NaHCO3) ___Titanium(Ti) ___Concrete Part4:Matcheachdiagramwithitscorrectdescription.Diagramswillbeusedonce. A B C D E ____1.PureElement–onlyonetypeofatompresent. ____2.Mixtureoftwoelements–twotypesofuncombinedatomspresent. ____3.Purecompound–onlyonetypeofcompoundpresent. ____4.Mixtureoftwocompounds–twotypesofcompoundspresent. ____5.Mixtureofacompoundandanelement.