* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Synthetic biology wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Endomembrane system wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Signal transduction wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Proteolysis wikipedia , lookup
Protein adsorption wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Biosynthesis wikipedia , lookup
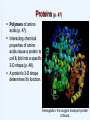
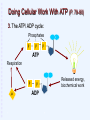
Welcome to Biology 121 “Concepts of Biology” Fall 2015 Page numbers correspond to the 3rd,4th & 5th edition of our text. WHAT IS BIOLOGY? Biology is the study of living Organisms Life at its Many Levels (p.17) The universe (and life) show levels of organization from simple to complex, small to large: * Some whole organisms are single-celled (bacteria, yeast) & thus “skip” the tissue, organ, & system levels. Unity of Life: Common Characteristics of all Living Things (p. 7) What characterizes living things? DNA: basis of Inheritance & reproduction. Obtain, process, & use energy via metabolism. Sense & respond to changes in environment. Maintain homeostasis: “A state where the internal environment is maintained within a range life can tolerate”. Reproduce Evolve (change) over generations (p. 10). Evolution: 'Life's Unifying Theme' --all life shares a common ancestry (p. 10-12) Modern Biology Stands on 4 Key Scientific theories: 1) The Cell theory: All organisms composed of 1 or more cells, etc. Basis for our understanding of cells, tissues, organs, growth, reproduction, etc. 2) The Central Dogma: Basis of heredity: Information stored in DNA, transcribed to RNA & used to generate proteins (= the 'drivers' of biological activity). 3) The Theory of Evolution by Natural Selection: Biological populations change (evolve) when genetic variability provides some individuals a higher chance of survival & reproduction in their particular environment. Explains biological diversity & ecological interactions. 4) The Ecosystem Concept: Living organisms interact with each other & their environment to form ecosystems. WHAT IS BIOLOGY? Biology is the study of living organisms The Process of Science p. 4-7 (14-16) Scientific Methods: Logical, repeatable approaches to explaining nature. “Evidence-based thinking” Observe aspect of nature & ask a question. Develop a hypothesis (tentative answer). Using hypothesis, make a prediction. Test prediction with experiments, observations, models, etc; evaluate & analyze results. Objectively report results & conclusions. Scientific theory: An accepted body of knowledge derived from multiple hypotheses that have withstood many testings. Part 1: The Cell Theory Chapters 2 & 3: Life’s Chemical Basis What are cells made of? Atoms (p 25). Atomic nucleus: protons (positive charge). neutrons (no charge). Electrons: Suround atomic nucleus. negative charge. Molecules: 2 or more atoms that have reacted & bonded together (p. 27). Various types of chemical bonds may form between atoms: O H H A water molecule: 2 hydrogens bonded to oxygen Important Chemicals for Life Water: 2-hydrogens & 1-oxygen. majority of living matter (p. 29-31) . Other “inorganic” molecules ions + or – charged (have gained or lost electrons). Salts (release ions when dissolved) Others (acids, bases, etc) p. 31-32. Organic molecules (carbon-based): Chapter 3 Many kinds, often large, & elaborate Organic Molecules: The Molecules of Life (Ch. 3) Carbon-based molecules (p. 38): Carbon "backbone" Hydrogen Other elements (O, N, S, P, etc.) 4 main types Hydrogen HHHHH O=C-C-C-C-C-H HHHH Other elements (often O, N, P, or S) Carbon “backbone” Carbon’s Versatile Bonding Behavior: (p. 38) Carbon can form 4 different bonds: Result: Complex structures are possible: Glucose Giant Molecules from Smaller Building Blocks: Polymerization (p. 39) Cells often link many small organic molecules together: Monomers: basic subunits. Polymers: large molecule chains composed of many monomers. dehydration reaction LEU LYS GLT Monomers LEU GLT hydrolysis reaction LYS LYS Polymer LEU A LA Organic Molecules: Carbohydrates (p. 40) Most abundant organic in nature. (Carbon, Hydrogen, oxygen in 1:2:1 ratio) Exist as simple sugars or as polymers. Simple sugars: 3-carbon sugars (glyceraldehyde) Pentoses (5C) (ribose) Hexoses (6C) (glucose, fructose) Uses: Energy source, building blocks for other molecules. Carbohydrates: Disaccharides: 2 linked simple sugars (p. 41). Sucrose (=table sugar): Nature’s most plentiful sugar! Polysaccharides: (p. 42) (many sugars in a polymer) Starch: Plant food storage. Glycogen: Animal food storage. Part of a polysaccharide Structural Polysaccharides Cellulose: Plant cell walls (p.41) Chitin: External skeletons of some animals (insects, etc). Fungal cell walls. Centipede: Chitinreinforced body. Wood: Contains cellulose Fungus: Chitin cell walls. Lipids (p. 43): Greasy/ oily; not soluble in water Mostly just carbon & hydrogen. Functions: Energy storage (more than 2x energy/gram than carbohydrates). Cell membranes. Certain hormones. Types of Lipids: Fats and oils (p. 43-44): Structure: 3 fatty acids + glycerol (= Triglyceride) Saturated (no double bonds) vs. saturated Important for energy storage. Saturated fatty acid Unsaturated fatty acid Types of Lipids (Continued): Steroids: (p. 45). stabilize membranes (Cholesterol, etc) steroid hormones. Waxes: repel water, lubricate. Phospholipids: (p. 60; chapter. 4). 2 fatty acids + 1 phosphate + glycerol Important part of cell membranes Water soluble Not water soluble Proteins & Amino Acids (p. 46): Amino acids (p. 46): Small organic molecules, contain nitrogen 20 types. Some of the 20 Amino Acids Proteins (p. 47) Polymers of amino acids (p. 47). Interacting chemical properties of amino acids cause a protein to coil & fold into a specific 3-D shape (p. 48). A protein's 3-D shape determines it’s function. Hemoglobin: the oxygen transport protein of blood. Proteins: the Most Diverse Organic Molecules (p.46): Structural Proteins: “The stuff of spider webs or feathers, bone, hair & other body parts”. Enzymes: Enable (speed up) biochemical reactions. Nutritious (storage) proteins: seeds, eggs, etc. Transport proteins: transport substances in cells. Contractile proteins: Movement, muscles! Metabolism is Controlled by Proteins! Metabolism: The Cell's many chemical reactions. Metabolic pathway: An orderly series of reactions: Each step mediated by a particular enzyme (p. 46) Thousands of metabolic pathways the body. Thousands of enzymes needed! Enzymes: Catalyze Biochemical Reactions in Cells (p. 80-82 in Ch 5) 3-D shape is important for enzyme function. Lock and Key model (p.82): substrate Enzyme Product Enzyme - substrate complex Nucleotides & Nucleic Acids (p. 49) Nucleotides: Small organic molecules with a phosphate* + a 5-C sugar + a nitrogen-containing base. Functions: Building blocks for DNA & RNA (p. 49-50). Energy carrier: ATP. Base (with N) Others. Phosphate Sugar * Phosphate: a chemical group that contains the element phosphorus Diagram of a nucleotide ATP: The Cell’s Energy Battery (p. 79-80 in Ch. 5) Problem: Many reactions require energy. Solution: Couple them with an energy-releasing reaction! 1. Cells use respiration (= fuel consumption) to release energy. 2. This energy used to make ATP. p p p ATP Doing Cellular Work With ATP (P. 79-80) 3. The ATP/ ADP cycle: Phosphates p p p ATP Respiration p p Released energy, biochemical work p ADP Nucleic Acids: Polymers of Nucleotides (P. 49-50) DNA 2 antiparallel strands made of 4 types of nucleotides. Nucleotide bases “pair” with those on opposite strand. Double helix (spiral). Combinations of thousands of base-pairs store genetic information: Blueprint for cell’s proteins. RNA Single strand of nucleotides Several types; Transmit DNA instructions to cell. 3´ 5´ A T G C T A C G 3´ 5´ The End Version 15.09