* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download aminoacids 2
Protein–protein interaction wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Human digestive system wikipedia , lookup
Western blot wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Citric acid cycle wikipedia , lookup
Point mutation wikipedia , lookup
Peptide synthesis wikipedia , lookup
Wilson's disease wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Protein structure prediction wikipedia , lookup
Genetic code wikipedia , lookup
Proteolysis wikipedia , lookup
Biosynthesis wikipedia , lookup
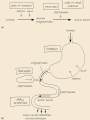
Faculty of nursing CHEM 203 Biochemistry UNIT VII Amino acids , Protein chemistry and metabolism Part Dr. Ola Fouad Talkhan The process of digestion is defined as the ‘process by which macromolecules in food are broken down into their component smallmolecule subunits’. The macromolecules are the proteins or polypeptides themselves, and the subunits are the amino acids. The bonds holding the subunits together are peptide bonds the degradation of proteins by cellular enzymes enzymes in a process called hydrolysis. Protein digestion takes place in two different phases: ◦ In the stomach ◦ In the small intestine Both of these phases of digestion are based on several types of enzymes that are called proteinases and proteases. Gastrin -stimulates Parietal cells to secrete HCL; Chief cells of the gastric glands to secrete pepsinogen Hydrochloric acid -Denatures protein structure -Activates pepsinogen (zymogen) to pepsin Pepsin -hydrolyzes proteins to smaller polypeptides and some free amino acids. Proteins Pepsin , H+ pH 1-2 proteoses + peptones The remainder of protein digestion occurs in the small intestine as the result of the action of enzymes such as 1- trypsin , chemotrypsin and carboxypeptidase (secreted by the pancreas) proteoses peptones Trypsin, chemotrypsin HCO3 , pH 8.0 polypeptides + A.A. aminopeptidase polypeptides di, and tripeptidase + A.A. carboxypeptidase 2- peptidases ( aminopeptidase ,dipeptidase and tripeptidase ) (located in the cells that line the small intestine). di, and tripeptidase dipeptidase tripeptidase A.A. Proteins are broken down to ◦ Tripeptides ◦ Dipeptides ◦ Free amino acids Free amino acid small intestine(villi)Liverblood circulation Form in which the majority of protein is absorbed More rapid than absorption of free amino acids Active transport ◦ Energy required Metabolized into free amino acids in enterocyte Only free amino acids absorbed into blood Amino Acid Degradation and Synthesis The catabolism of the amino acids involves the removal of α-amino groups (NH3), followed by the breakdown of the resulting carbon skeletons. These pathways converge to form seven intermediate products:oxaloacetate, pyruvate, α-ketoglutarate, fumarate, succinyl coenzyme A (CoA), acetyl CoA, and acetoacetate. these will enter in the synthesis of glucose or lipid or in the production of energy through their oxidation to CO2 by the citric acid cycle Nonessential amino acids can be synthesized from the intermediates of metabolism or, as in the case of cysteine and tyrosine, from essential amino acids Fate of NH3 produced in deamination Ammonia produced from deamination of A.A. are very toxic to the brain and nervous tissues so, it is rapidly removed by : 1. Urea : Formation of urea in the liver is quantitatively the most important disposal route for ammonia. Urea travels in the blood from the liver to the kidneys, where it passes into the glomerular filtrate. 2. Glutamine synthesis in brain and liver Circulating glutamine is removed by the liver and the kidneys and deaminated by glutaminase. In the liver, the NH3 produced is detoxified through conversion to urea , and in the kidney it can be used in the excretion of protons. Hyperammonemia The capacity of the hepatic urea cycle exceeds the normal rates of ammonia generation, and the levels of serum ammonia are normally low (5–35 μmol/L). However, when liver function is compromised, due either to genetic defects of the urea cycle or liver disease, blood levels can rise above 1,000 μmol/L. Such hy per ammon emia is a medical emergency, because ammonia has a direct neurotoxic effect on the CNS the symptoms of ammonia intoxication, 1-tremors, slurring of speech, somnolence, vomiting, cerebral edema, and blurring of vision. 2-At high concentrations, ammonia can cause coma and death. The two major types of hyperammonemia are: 1. Acquired hyperammonemia: Liver disease is a common cause of hyperammonemia in adults, and may be due, for example, to viral hepatitis or to hepatotoxins such as alcohol 2. Congenital hyperammonemia: Genetic deficiencies of each of the five enzymes of the urea cycle have been described METABOLIC DEFECTS IN AMINO ACID METABOLISM 1- Phenylketonuria (PKU). Phenylketonuria (PKU), caused by a deficiency of phenylalanine hydroxylase A deficiency in phenylalanine hydroxylase results in the disease phenylketonuria (PKU). Characteristics of classic PKU: 1-Elevated phenylalanine:Phenylalanine is present in elevated concentrations in tissues, plasma, and urine. 2-CNS symptoms: Mental retardation, failure to walk or talk, seizures, hyperactivity, tremor, microcephaly, and failure to grow are characteristic findings in PKU. 3-Hypopigmentation: Patients with phenylketonuria often show a deficiency of pigmentation (fair hair, light skin color, and blue eyes). 2- Maple syrup urine disease (MSUD) is a rare (1:185,000), autosomal recessive disorder in which there is a partial or complete deficiency in branched-chain α-keto acid dehydrogenase, an enzyme complex that decarboxylates leucine, isoleucine, and valine Accumulation of these A.A. in the blood, causing a toxic effect that interferes with brain functions. Characteristics of Maple syrup urine disease feeding problems, vomiting, dehydration, severe metabolic acidosis, and a characteristic maple syrup odor to the urine. If untreated, the disease leads to mental retardation, physical disabilities, and even death. 3- Homocystinuria The homocystinurias are a group of disorders involving defects in the metabolism of homocysteine. The diseases are inherited as autosomal recessive illnesses,due to a defect in the enzyme cystathionine β-synthase, Characteristics of Homocystinuria high plasma and urinary levels of homocysteine and methionine and low levels of cysteine. ectopia lentis (displacement of the lens of the eye), skeletal abnormalities, a tendency to form thrombi (blood clots), osteoporosis, and neurological deficits. Patients can be responsive or nonresponsive to oral administration of pyridoxine (vitamin B6)