* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Type I cell death Apoptosis
Survey
Document related concepts
Transcript
Molecular mehanim of cell death
By:
Sundus Hafeez
Rubia ain
Momina Masud
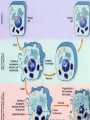
CELL DEATH
Major cause of cell death:
This is due to irreversible cell injury with continuing cell
damage, resulting in morphologic changes that can be called
as “cell death”
Reasons may be
Hypoxia
Physical agents
Chemical agents
Infectious agents
Genetic imbalances etc
Basically three types
Apoptosis= suicide - programmed cell death
2. Necrosis= killing - decay and destruction
3. Autophagy= cell degradation through cell’s own lysosomal machinery
1.
NECROSIS
It results in the premature death of cells in living tissue
and unregulated digestion of cell components
Occurs due to extrinsic factors infection, toxins, or trauma
almost always detrimental and can be fatal
result in the loss of cell membrane integrity and an
uncontrolled release of products of cell death into the
intracellular space
Necrosis results in:
an inflammatory response in the surrounding tissue
Prevention of phagocytes from locating and engulfing dead cells
build-up of dead tissue and cell debris at, or near, the site of the cell
death
NECROSIS TYPES
One of these five macroscopic changes is observed due to necrosis
1.
2.
3.
4.
5.
Coagulative necrosis: the outline of the dead cells are maintained
and the tissue is firm (due to loss of blood supply)
Liquefactive necrosis: the dead cells undergo disintegration and
affected tissue is liquefied.
Caseous necrosis: combination of coagulative+liquefactive
necrosis, resulting in cheese-like mass
Fat necrosis: enzymatic digestion of fat by pancreatic enzymes
Fibrinoid necrosis: a special form of necrosis usually caused by
immune-mediated vascular damage, marked by formation of
immune complexes
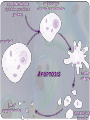
APOPTOSIS
• The actual "suicide" of the cell which results in
engulfment of the cell remains by specialized immune
cells called phagocytes;
degradation of engulfed cell.
• This process helps to eliminate unwanted cells by an
internally programmed series of events
• Balance is maintained between cell proliferation and
death
It occurs during:
During development for removal of excess cells during embryogenesis
To maintain cell population in tissues with high turnover of cells, such as skin,
bowels
To eliminate immune cells after cytokine depletion, and autoreactive T-cells in
developing thymus.
Hormone-dependent involution - Endometrium, ovary, breasts, atrophy of ovary
during menopause etc.
To remove damaged cells by virus
To eliminate cells after DNA damage by radiation, cytotoxic agents etc.
Cell death in tumors.
APOPTOSIS MORPHOLOGY
1.
2.
3.
4.
5.
Shrinkage of cells
Condensation of nuclear chormatin peripherally under
nuclear membrane
Formation of apoptotic bodies by fragmentation of the cells
and nuclei. The fragments remain membrane-bound
Phagocytosis of apoptotic bodies by adjacent healthy cells
or phagocytes
Unlike necrosis, apoptosis is not accompanied by
inflammatory reaction
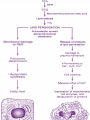
Autophagy
Autophagy is a self-digesting mechanism responsible for
removal of damaged organelles, malformed proteins during
biosynthesis, and nonfunctional long-lived proteins by
lysosome.
Types
Autophagy has been divided into three general types.
microautophagy
chaperone-mediated autophagy (CMA),
macroautophagy.
Microautophagy :
cytoplasm material is sequestered through direct invagination to
the lysosomal membrane.
CMA:
proteins flagged with pentapeptide motif (KFERQ) were
selectively degraded through direct translocation into lysosome.
Macroautophagy :
formation of subcellular double-membrane-bound structures
called autophagosomes that contain degradable contents of cytoplasm
materials and deliver them into lysosomes for breakdown by lysosomal
enzymes.
Autophagy begins with the
formation of double-membranebounded autophagosomes.
The mammalian target of
rapamycin (mTOR) is a negative
regulator of autophagosome
formation.
Autophagosomes fuse with
lysosomes to form
autophagolysosomes,
The contents of
autophagolysosomes are finally
degraded by acidic lysosomal
hydrolases.
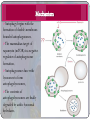
Type I cell death
Apoptosis mechanism
MECHANISM
Apoptosis Triggered via Two Pathways
Intrinsic or mitochondrial pathway
Extrinsic or death receptor pathway
Extrinsic pathway
• Binding of Fas by FasL induces recruitment of
FADD to the cytoplasmic tail of Fas
• The opposite end of FADD contains a death
effector domain (hatched boxes); recruitment
of either procaspase-8 or c-FLIP
• Caspase-8 can cleave Bid
• truncated Bid (tBid) can inactivate Bcl-2 in the
mitochondrial membrane.
• This allows the escape of cytochrome c, which
clusters with Apaf-1 and caspase-9 in the
presence of dATP to activate caspase-9.
• Smac/DIABLO is also released from the
mitochondria and inactivates inhibitors of
apoptosis (IAPs).
• breakdown of several cytoskeletal proteins and
degradation of the inhibitor of caspase-activated
DNase (ICAD).
Intrinsic pathway
In a healthy cell, the outer membranes of its mitochondria display the protein
Bcl-2 on their surface.
Internal damage to the cell (e.g., from reactive oxygen species) causes
Bcl-2 to activate a related protein, Bax, which punches holes in the outer
mitochondrial membrane, causing
cytochrome c to leak out.
The released cytochrome c binds to the protein Apaf-1 ("apoptotic protease
activating factor-1").
Using the energy provided by ATP,
these complexes aggregate to form apoptosomes.
The apoptosomes bind to and activate caspase-9.
Caspase-9 cleaves and, in so doing, activates other caspases (caspase-3 and -7).
The activation of these "executioner" caspases creates an expanding cascade of
proteolytic activity which leads to
digestion of structural proteins in the cytoplasm,
degradation of chromosomal DNA, and
phagocytosis of the cell.
Necrosis and its Mechanism
Necrosis has been defined as a type of cell death that lacks the
features of apoptosis and is usually considered to be
uncontrolled.
After signaling- or damage-induced lesions, necrosis can include signs
of controlled processes such as mitochondrial dysfunction, enhanced
generation of reactive oxygen species, ATP depletion and early plasma
membrane rupture.
The inhibition of specific proteins involved in regulating apoptosis or
autophagy can change the type of cell death to necrosis.
A classical definition of necrosis based on morphological criteria(early
plasma membrane rupture and dilatation of cytoplasmic organelles, in
particular mitochondria).
necrosis is often associated with unwarranted cell loss in human
pathologies and can lead to local inflammation, presumably through
the liberation of factors from dead cells that alert the innate immune
system
apoptotic cells (which shrink) are engulfed completely by phagocytes,
necrotic cells (which swell) are internalized by a macropinocytotic
mechanism.
meaning that only parts of the cell are taken up by phagocytes
necrotic cell death can be a regulated event that contributes to
development and to the maintenance of organismal homeostasis.
Programmed cell necrosis can be a consequence of extracellular
signaling or can be initiated as a form of cellular suicide in response to
intracellular perturbations.
programmed cell necrosis plays a role in a number of disease
processes including vascular-occlusive disease, neurodegenerative
diseases, infection, inflammatory diseases, exposures to toxins, and
cancer
The core events of necrosis are bioenergetic failure and rapid loss of
plasma membrane integrity.
These can result from defined molecular events that occur in the dying
cell, including increased mitochondrial ROS production, channelmediated calcium uptake, activation of nonapoptotic proteases, and/or
enzymatic destruction of cofactors required for ATP production.
DNA damage-induced necrosis occurs selectively in growing cells.
DNA damage-induced necrosis occurs selectively in growing cells. Highly
proliferative cells utilize aerobic glycolysis for ATP production as other nutrient
sources such as amino acids and lipids are redirected into synthetic reactions
that support cell growth and proliferation. In response to alkylating DNA
damage, the nuclear enzyme PARP is activated and degrades NAD into
poly(ADP)-ribose polymers and nicotinic acid mononucleotide (NAM). The
consumption of NAD depletes the cellular NAD pool and shuts down the cell's
ability to degrade glucose to support ATP production, leading to necrosis.
Calcium-mediated programmed necrosis.
Calcium-mediated programmed necrosis. Intracellular calcium increases in
response to the activation of ionotrophic glutamate receptors or through other
calcium channels on the plasma membrane or the ER membrane. An
intracellular calcium spike induces the activation of Ca2+-dependent
proteases and stimulates mitochondrial TCA cycle activity and ROS
production. If sustained, the resulting ROS leads to mPT that is dependent on
CypD. mPT then leads to the loss of ATP production and necrosis.
Clearance of apoptotic and necrotic cells
and its immunological consequences
The ultimate and most favorable fate of almost all dying cells is
engulfment by neighboring or specialized cells.
apoptotic cells engulfment is regulated by a system of receptors on the
phagocytic cells.
the phagocytic cells detect molecules specific for dying cells.
clearance of dying cells is an important fundamental process serving
the regulation of normal tissue homeostasis.
cell corpses may release cytotoxic substances due to which there are
phagocytosed.
binding or uptake of apoptotic cells to phagocytes induces production
of transforming growth factor β (TGF-β) and sometimes interleukin10.
These anti-inflammatory cytokines have direct autocrine and paracrine
effects on proinflammatory cytokine production.
Apoptotic cell uptake stimulates lipid mediators such as 15lipoxygenase and 15-hydroxyeicosatetraenoic acid.
This enhances uptake of apoptotic cells by phagocytes.
Nonprofessional phagocytes such as endothelial or epithelial cells that
phagocytose neighboring apoptotic cells subsequently produce survival
and growth factors.
These include vascular endothelial growth factor and hepatocyte
growth factor.
They probably contribute to tissue replenishment and restoration of
endothelial and epithelial boundaries.
early apoptotic cells can be cleared silently without release of either
pro- or anti-inflammatory mediators.
Apoptotic cell uptake predominantly initiates mechanisms that
contribute to resolution of injury and repair.
but this must be seen in the context of other signals that impinge on
the surface receptors of phagocytes.
Necrotic cells and pathogens share many of the ligands of apoptotic
cells.
They usually induce different responses at least partially because they
also engage pattern recognition receptors and signaling pathways not
activated by apoptotic corpses.
apoptotic cell uptake does not immediately switch individual
phagocyte function.
only does so after a critical number of cells have contributed to an
overall change in the microenvironment.