* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Classifying Chemical Reactions by What Atoms Do
Determination of equilibrium constants wikipedia , lookup
Citric acid cycle wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Chemical bond wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Marcus theory wikipedia , lookup
Asymmetric induction wikipedia , lookup
Water splitting wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Rate equation wikipedia , lookup
Electrolysis of water wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Extended periodic table wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Transition state theory wikipedia , lookup
Thermometric titration wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Stoichiometry wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Click chemistry wikipedia , lookup
Oxidation state wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Acid–base reaction wikipedia , lookup
Chemical reaction wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Electrochemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Classifying Chemical Reactions by
What Atoms Do
Classification of Reactions
Synthesis reaction
Decomposition reaction
Single displacement reaction
Double displacement reaction
Synthesis Reactions
+
4 Al (s) + 3 O2 (g)
2 H2 (g) + O2 (g) --------->
C2H4 (g) + H2O2 (aq)
2 Al2O3 (s)
2 H2O (g)
C2H6O2 (l)
Decomposition Reactions
+
2 HgO (s) --------->
2 Hg (l) + O2 (g)
CaCO3 (s) ---------> CaO (s) + CO2 (g)
2 NaCl (s) --------->
Cl2 (g) + 2 Na (l)
Single Displacement Reactions
+
+
Cu (s) + 2 AgNO3 (aq) ---------> 2 Ag (s) + Cu(NO3)2 (aq)
2 Al (s) + Fe2O3 (s) ---------> Al2O3 (s) + 2 Fe (l)
Mg (s) + 2 HCl (aq) ---------> H2 (g) + MgCl2 (aq)
Double Displacement Reactions
+
+
Ba(NO 3)2 (aq) + Na2SO4 (aq) ---------> BaSO4 (s) + 2 NaNO 3 (aq)
PCl3 (l) + 3 AgF (s) ---------> PF3 (g) + 3 AgCl (s)
HCl (aq) + NaOH (aq) ---------> H2O(l) + NaCl (aq)
Chemical Reactions Classified by Reaction Type
Precipitation
Reactions
Precipitation Reactions
Precipitation reactions are reactions in which a
solid forms when we mix two solutions.
1) reactions between aqueous solutions of ionic compounds
2) produce an ionic compound that is insoluble in water
3) The insoluble product is called a precipitate.
Precipitation Reactions
2 KI(aq) + Pb(NO3)2(aq) ➜ PbI2(s) + 2 KNO3(aq)
No Precipitate Formation = No Reaction
KI(aq) + NaCl(aq) ➜ KCl(aq) + NaI(aq)
No precipitate forms, therefore, no reaction.
KI(aq)
KCl(aq) + NaI(aq)
NaCl(aq)
Process for Predicting the Products of
a Precipitation Reaction
1.
Determine which ions are present in each aqueous reactant.
2.
Determine formulas of possible products.
3.
Determine solubility of each potential product in water.
4.
If neither product will precipitate, write no reaction after the
arrow.
5.
If any of the possible products are insoluble, write their formulas as
the products of the reaction using (s) after the formula to indicate
solid. Write any soluble products with (aq) after the formula to
indicate aqueous.
6.
Balance the equation.
Remember to only change coefficients, not subscripts
Predict the products and balance the
equations
K2CO3(aq) + NiCl2(aq) ➜
K2CO3(aq) + NiCl2(aq) ➜
KCl (?) + NiCO3(?)
K2CO3(aq) + NiCl2(aq) ➜ 2 KCl (?) + NiCO3(?)
K2CO3(aq) + NiCl2(aq) ➜ 2 KCl (aq) + NiCO3(s)
Predict the products and balance the
equations
KCl(aq) + AgNO3(aq) ➜
KCl(aq) + AgNO3(aq) ➜ KNO3(?) + AgCl(?)
KCl(aq) + AgNO3(aq) ➜ KNO3(aq) + AgCl(s)
Predict the products and balance the
equations
Na2S(aq) + CaCl2(aq) ➜
Na2S(aq) + CaCl2(aq) ➜ NaCl(?) + CaS(?)
Na2S(aq) + CaCl2(aq) ➜ 2 NaCl(?) + CaS(?)
Na2S(aq) + CaCl2(aq) ➜ 2 NaCl(aq) + CaS(aq)
No Reaction !!!!
Predict the products and balance the
equations
(NH4)2SO4(aq) + Pb(C2H3O2)2(aq) ➜
(NH4)2SO4(aq) + Pb(C2H3O2)2(aq) ➜ NH4C2H3O2(?) + PbSO4(?)
(NH4)2SO4(aq) + Pb(C2H3O2)2(aq) ➜ 2 NH4C2H3O2(?) + PbSO4(?)
(NH4)2SO4(aq) + Pb(C2H3O2)2(aq) ➜ 2 NH4C2H3O2(aq) + PbSO4(s)
Ionic Equations
Equations that describe the chemicals put into the water and
the product molecules are called molecular equations.
2 KOH(aq) + Mg(NO3)2(aq) ➜ 2 KNO3(aq) + Mg(OH)2(s)
Equations that describe the material’s structure when
dissolved are called complete ionic equations.
Aqueous strong electrolytes are written as ions.
Insoluble substances, weak electrolytes, and nonelectrolytes are
written as molecules.
2K+(aq) + 2OH−(aq) + Mg2+(aq) + 2NO3−(aq) ➜ 2K+(aq) + 2NO3−(aq) + Mg(OH)2(s)
Ionic Equations
Ions that are both reactants and products are called spectator ions.
2 K+(aq) + 2 OH−(aq) + Mg2+(aq) + 2 NO3−(aq) ➜ 2 K+(aq) + 2 NO3−(aq) + Mg(OH)2(s)
An ionic equation in which the spectator ions are removed is called a
net ionic equation.
2 OH−(aq) + Mg2+(aq) ➜ Mg(OH)2(s)
Write the ionic and net ionic equation
K2SO4(aq) + 2 AgNO3(aq) ➜ 2 KNO3(aq) + Ag2SO4(s)
2K+ (aq) + SO42-(aq) + 2Ag+ (aq) + 2NO3-(aq) ➜ 2K+ (aq) + 2NO3-(aq) + Ag2SO4(s)
2 Ag+(aq) + SO42−(aq) ➜ Ag2SO4(s)
Write the ionic and net ionic equation
Na2CO3(aq) + 2 HCl(aq) ➜ 2 NaCl(aq) + CO2(g) + H2O(l)
2Na+ (aq) + CO32-(aq) + 2H+ (aq) + 2Cl-(aq) ➜ 2Na+ (aq) + 2Cl-(aq) + CO2(g) + H2O(l)
CO32−(aq) + 2 H+(aq) ➜ CO2(g) + H2O(l)
Acids and Bases
Acids and Bases in Solution
Acids ionize in water to form H+ ions.
(More precisely, the H+ from the acid molecule is donated
to a water molecule to form hydronium ion, H3O+)
Bases dissociate in water to form OH- ions.
(Bases, such as NH3, that do not contain OH- ions,
produce OH- by pulling H+ off water molecules.)
In the reaction of an acid with a base, the H+ from the acid
combines with the OH- from the base to make water.
The cation from the base combines with the anion from the
acid to make a salt.
acid + base ➜ salt + water
Molecular Models of Selected Acids
Acid-Base Reactions
Also called neutralization reactions because the
acid and base neutralize each other’s properties
2 HNO3(aq) + Ca(OH)2(aq) ➜ Ca(NO3)2(aq) + 2 H2O(l)
Note that the cation from the base combines with the
anion from the acid to make the water soluble salt.
The net ionic equation for an acid-base reaction is
H+(aq) + OH-(aq) ➜ H2O(l)
(as long as the salt that forms is soluble in water)
Common Acids
Common Bases
HCl(aq) + NaOH(aq) ➜ NaCl(aq) + H2O(l)
HCl(aq)
NaOH(aq)
NaCl(aq) +
H2O(l)
Write the molecular, ionic, and net-ionic
equation for the acid-base reaction
HNO3(aq) + Ca(OH)2(aq) ➜
HNO3(aq) + Ca(OH)2(aq) ➜ Ca(NO3)2(aq) + H2O(l)
2HNO3(aq) + Ca(OH)2(aq) ➜ Ca(NO3)2(aq) + 2H2O(l)
2H+ (aq) + 2NO3-(aq) + Ca2+ (aq) + 2OH-(aq) ➜ Ca2+ (aq) + 2NO3-(aq) + 2H2O(l)
2H+(aq) + 2OH-(aq) ➜ 2H2O(l)
Write the molecular, ionic, and net-ionic
equation for the acid-base reaction
HCl(aq) + Ba(OH)2(aq) ➜
HCl(aq) + Ba(OH)2(aq) ➜ BaCl2(aq) + H2O(l)
2HCl(aq) + Ba(OH)2(aq) ➜ BaCl2(aq) + 2H2O(l)
2H+ (aq) + 2Cl-(aq) + Ba2+ (aq) + 2OH-(aq) ➜ Ba2+ (aq) + 2Cl-(aq) + 2H2O(l)
2H+(aq) + 2OH-(aq) ➜ 2H2O(l)
Write the molecular, ionic, and net-ionic
equation for the acid-base reactions .
H2SO4(aq) + Sr(OH)2(aq) ➜
H2SO4(aq) + Sr(OH)2(aq) ➜ SrSO4(s) + 2 H2O(l)
2H+ (aq) + SO42-(aq) + Sr2+ (aq) + 2OH-(aq) ➜ SrSO4 (s) + 2H2O(l)
2H+(aq) + SO42-(aq) + Sr2+ (aq) + 2OH-(aq) ➜ SrSO4 (s) + 2H2O(l)
Titration
A solution’s concentration is determined by
reacting it with another solution and using
stoichiometry – this process is called titration.
In the titration, the unknown solution is added to
a known amount of another reactant until the
reaction is just completed. At this point, called
the endpoint, the reactants are in their
stoichiometric ratio.
The unknown solution is added slowly from an
instrument called a burette.
Acid-Base Titrations
The difficulty is determining when there has
been just enough titrant added to complete
the reaction.
In acid-base titrations, because both the
reactant and product solutions are colorless,
a chemical (indicator) is added that changes
color when the solution undergoes large
changes in acidity/alkalinity
At the endpoint of an acid-base titration, the
number of moles of H+ equals the number of
moles of OH-(equivalence point).
Titration
The titrant is the base
solution in the burette.
As the titrant is added to
the flask, the H+ reacts
with the OH– to form
water. But there is still
excess acid present so the
color does not change.
At the titration’s endpoint,
just enough base has been
added to neutralize all the
acid. At this point the
indicator changes color.
Titration
The titration of 10.00 mL of HCl solution of unknown concentration
requires 12.54 mL of 0.100 M NaOH solution to reach the end point.
What is the concentration of the unknown HCl solution?
HCl(aq) + NaOH(aq) ➜ NaCl(aq) + H2O(l)
mL
NaOH
L
NaOH
mL
HCl
L
HCl
mol
NaOH
mol
HCl
The titration of 10.00 mL of HCl solution of unknown concentration
requires 12.54 mL of 0.100 M NaOH solution to reach the end point.
What is the concentration of the unknown HCl solution?
HCl(aq) + NaOH(aq) ➜ NaCl(aq) + H2O(l)
= 1.25 x 10-3 mol HCl reacted
What is the concentration of NaOH solution that
requires 27.5 mL to titrate 50.0 mL of 0.1015 M H2SO4 ?
H2SO4 (aq) + 2 NaOH (aq) ➜ Na2SO4 (aq) + 2 H2O (l)
L H2SO4
mol H2SO4
mol NaOH
M NaOH
L NaOH
reacted
Gas-Evolving
Reactions
Gas-Evolving Reactions
Some reactions form a gas directly from the ion exchange:
K2S(aq) + H2SO4(aq) ➜ K2SO4(aq) + H2S(g)
Other reactions form a gas by the decomposition of one
of the ion exchange products into a gas and water.
K2SO3(aq) + H2SO4(aq) ➜ K2SO4(aq) + H2SO3(aq)
H2SO3 ➜ H2O(l) + SO2(g)
NaHCO3(aq) + HCl(aq) ➜ NaCl(aq) + CO2(g) + H2O(l)
NaHCO3(aq)
NaCl(aq) + CO2(g)
+ H2O(l)
HCl(aq)
NaHCO3(aq) + HCl(aq) ➜ NaCl(aq) + CO2(g) + H2O(l)
{NaHCO (aq) + HCl(aq) ➜ NaCl(aq) + H CO (aq)}
3
2
H2CO3 ➜ H2O(l) + CO2(g)
3
“carbonic
acid”
Na2CO3(aq) + 2 HCl(aq) ➜ 2 NaCl(aq) + CO2(g) + H2O(l)
Compounds that Undergo
Gas-Evolving Reactions
Practice – Predict the products and
balance the equations
Na2CO3(aq) + 2 HNO3(aq) ➜
2 NaNO3(aq) + H2O (l) + CO2(g)
2 HCl(aq) + Na2SO3(aq) ➜
2 NaCl (aq) + H2O (l) + SO2 (g)
H2SO4(aq) + CaS(aq) ➜
CaSO4(aq) + H2S(aq)
Redox Reactions
Oxidation/Reduction Basic Definitions
Oxidation and Reduction - Symbolic Representation
Oxidation and Reduction at the Atomic Level
Redox Reactions
Oxidation/reduction reactions involve transferring
electrons from one atom to another.
Also known as redox reactions
Many involve the reaction of a substance with O2(g).
4 Fe(s) + 3 O2(g) ➜ 2 Fe2O3(s)
Atoms in Elements-------> Ions in Compound
Combustion as Redox
2 H2(g) + O2(g) ➜ 2 H2O(g)
Redox without Combustion
2 Na(s) + Cl2(g) ➜ 2 NaCl(s)
Reactions of Metals with Nonmetals
Consider the following reactions:
4 Na(s) + O2(g) → 2 Na2O(s)
2 Na(s) + Cl2(g) → 2 NaCl(s)
The reactions involve a metal reacting with a nonmetal.
In addition, both reactions involve the conversion of free
elements into ions.
Na2O = 2 Na+ + O2NaCl = Na+ + Cl-
Oxidation and Reduction
To convert a free element into an ion, the atoms
must gain or lose electrons (of course, if one
atom loses electrons, another must accept
them).
Atoms that lose electrons are being oxidized,
atoms that gain electrons are being reduced.
2 Na(s) + Cl2(g) → 2 Na+Cl–(s)
Na → Na+ + 1 e– oxidation
Cl2 + 2 e– → 2 Cl– reduction
Electron Bookkeeping
For reactions that are not metal + nonmetal, or do not involve
O2, we need a method for determining how the electrons
are transferred.
Chemists assign a number to each element in a reaction called
an oxidation state that allows them to determine the
electron flow in the reaction.
Even though they look like them, oxidation states
are not ion charges!
Oxidation states are imaginary charges assigned
based on a set of rules.
Ion charges are real, measurable charges.
Rules for Assigning Oxidation States
(in order of priority)
1. Free elements have an oxidation state = 0.
In Na (s), Na = 0 ; In Cl2 (g), Cl2 = 0
2. Monatomic ions have an oxidation state equal to
their charge.
In NaCl, Na = +1 and Cl = −1
3. (a) The sum of the oxidation states of all the atoms
in a compound is 0.
Na = +1 and Cl = −1 in NaCl, (+1) + (−1) = 0
Rules for Assigning Oxidation States
(in order of priority)
3.
(b) The sum of the oxidation states of all the atoms in a
polyatomic ion equals the charge on the ion.
In NO3–, N = +5 and O = −2 [3 x (2-) + 1 x (5+) = -1]
4.
(a) Group I metals have an oxidation state of +1 in all their
compounds.
(b) Group II metals have an oxidation state of +2 in all their
compounds.
Rules for Assigning Oxidation States
(in order of priority)
5. In their compounds, nonmetals have oxidation
states according to the table below
Assign an oxidation state to each
element in the following
Br2
Br = 0, (Rule 1)
K+
K = +1, (Rule 2)
LiF
Li = +1, (Rule 4a) & F = −1, (Rule 5)
CO2
O = −2, (Rule 5) & C = +4, (Rule 3a)
SO42−
O = −2, (Rule 5) & S = +6, (Rule 3b)
Na2O2
Na = +1, (Rule 4a) & O = −1 , (Rule 3a)
Determine the oxidation states of all the
atoms in a propanoate ion, C3H5O2–
There are no free elements or free ions in
propanoate, so the first rule that applies is
Rule 3b
(C3) + (H5) + (O2) = −1
Because all the atoms are nonmetals, the next
rule we use is Rule 5, following the elements
in order:
H = +1
O = −2
(C3) + 5(+1) + 2(−2) = −1
(C3) = −2
C = −⅔
Oxidation and Reduction
Another Definition
Oxidation occurs when an atom’s oxidation
state increases during a reaction.
Reduction occurs when an atom’s oxidation
state decreases during a reaction.
-4
0
CH4
+
oxidation
+4
-2
2 O2 → CO2 + 2 H2O
reduction
Oxidation–Reduction
Oxidation and reduction must
occur simultaneously.
2 Na(s) + Cl2(g) → 2 Na+Cl–(s)
Na is oxidized
Cl is reduced
Na is the reducing agent
Cl2 is the oxidizing agent
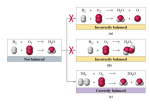
Assign oxidation states, determine the element oxidized
and reduced, and determine the oxidizing agent and
reducing agent in the following reactions:
4+
Sn
0
+ Ca →
2+
Sn
+
2+
Ca
Sn4+ is being reduced; Sn4+ is the oxidizing agent.
Ca is being oxidized; Ca is the reducing agent.
2
0
F2
0
+ S → SF4
S 4+
F-
F is being reduced from F0 to F-;F2 is the oxidizing agent.
S is being oxidized from S0 to S+4;S is the reducing agent.
Assign oxidation states, determine the element oxidized
and reduced, and determine the oxidizing agent and
reducing agent in the following reactions:
0
+7
+3
+4
Fe + MnO4− + 4 H+ → Fe3+ + MnO2 + 2 H2O
Reduction
Oxidation
Fe is the reducing agent.
MnO4− is the oxidizing agent.