* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Osmosis Experimental Design Lab
Survey
Document related concepts
Transcript
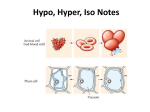
Biology HS/Science Unit: 04 Lesson: 02 Osmosis Experimental Design Lab Objective: Design an experiment to determine if starch and/or water can cross a simulated cell membrane. Background: Recall from discussions in class that cells use transport methods such as diffusion, osmosis, and active transport to allow substances to cross their cell membrane. Some transport methods are considered passive because they do not require the cell to expend any energy. Other transport methods are active because they require the cell to expend energy. In this lab, you will be investigating osmosis, the diffusion of water across a semi-permeable membrane. Three types of solutions can surround a cell: hypertonic, hypotonic, or isotonic. A hypertonic solution has more dissolved substances than inside the cell. A hypotonic solution has less dissolved substances than inside the cell. An isotonic solution has the same concentration of dissolved substances as inside the cell. The type of solution that surrounds a cell will influence the net flow of water and substances into and out of the cell. Pre-Lab Questions: 1. Diffusion is the net movement of substances from an area of _____________ concentration to an area of _______________ concentration. 2. A cell’s membrane is semi-permeable (also called “selectively permeable”). What does this mean? 3. If a cell is in a hypertonic solution, what is the direction of the net flow of water and why? 4. If a cell is in a hypotonic solution, what is the direction of the net flow of water and why? 5. If a cell is in an isotonic solution, what is the direction of the net flow of water and why? Experimental Design: Dialysis tubing is a semi-permeable membrane that acts the same way a cell membrane acts. During this lab, you will be using the dialysis tubing to create small baggies that will represent cells. The fluid inside and outside the baggies will be water and/or starch solution. Question: Which molecule is larger: starch or water? Your experimental design will be written to answer the following questions. In your science notebook, record your prediction for each question: 1. Can water cross the dialysis tubing by osmosis? 2. Can starch cross the dialysis tubing by diffusion? ©2012, TESCCC 08/16/12 page 1 of 3 Biology HS/Science Unit: 04 Lesson: 02 3. If there is a higher concentration of water inside the dialysis tubing baggie, what is the net movement of water? 4. If there is a higher concentration of water outside the dialysis tubing baggie, what is the net movement of water? 5. If the concentration of water is the same inside and outside the dialysis tubing baggie, what is the net movement of water? Materials: 250ml beakers (3) 1” dialysis tubing (40 cm) water 1% starch solution (300–400 mL) iodine (0.2 mL) scale or mm ruler paper towels string (12 in) 50 mL graduated cylinder timing device calculators Design an experiment to test your predictions using these materials. Write your experimental design in your science notebooks. Include a diagram showing each beaker/baggie system and what will be inside the beaker and baggie. Describe how and what you will measure during your experiment. Your teacher must approve your experimental design before you can conduct your experiment. When your experimental design is approved, set up your beakers and baggies. Dialysis Tubing Baggies: To make a baggie, soak the tubing in water for about one minute. Then, fold the bottom about 1 cm up, and tie the folded part with a small piece of string to create a bag. Then, open the other end of the tube by rubbing the end between your fingers until the edges are separated. You may want to blow into the bag to open it more and make pouring easier. After pouring the fluid into the baggie, leave some space to allow water to diffuse during your experiment. Next, fold 1 cm of tubing, and tie off the top of the tube with a small piece of string. Finally, rinse your tube and string in fresh water. Squeeze any extra water from the string. You will need to wait approximately 20 minutes to observe the effects of osmosis. While you are waiting, create a data table in your science notebook to record your data. After 20 minutes, collect and record your data. Then, write a summary of your results in your science notebook, and answer the following questions: 1. Were your predictions correct? If they were not correct, why do you think there was a difference between your predictions and what you observed during your experiment? 2. What do your results teach you about the effects of osmosis across a semi-permeable membrane? ©2012, TESCCC 08/16/12 page 2 of 3 Biology HS/Science Unit: 04 Lesson: 02 3. Explain what happens to an animal cell that is placed in a hypertonic solution vs. a hypotonic solution. 4. Explain what happens to a plant cell that is placed in a hypertonic solution vs. a hypotonic solution. 5. Discuss the percentage change inside and outside the baggie for your systems. ©2012, TESCCC 08/16/12 page 3 of 3