* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Oakland Schools Biology Resource Unit
Survey
Document related concepts
Basal metabolic rate wikipedia , lookup
Drug discovery wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Western blot wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Biosynthesis wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Blood sugar level wikipedia , lookup
Phosphorylation wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Transcript
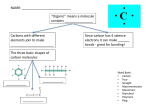
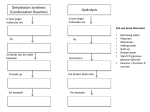
Oakland Schools Biology Resource Unit 1 Chemistry and Biochemistry Colleen Cain Avondale High School Avondale School District Unit Table of Contents Standards, Statements and Expectations Instructional Background Information Terms and concepts Knowledge and Skills Instructional Resources Activity #1: Who Took Jerrel’s iPod? Activity #2: Analyzing Food Labels Activity #3: Pineapple Enzymes and Protein Lab Activity #4: Dehydration Synthesis and Hydrolysis Virtual Simulation Standards, Statements and Expectations Standard B2: Organization and Development of Living Systems Students describe the general structure and function of cells. They can explain that all living systems are composed of cells and that organisms may be unicellular or multicellular. They understand that cells are composed of biological macromolecules and that the complex processes of the cell allow it to maintain a stable internal environment necessary to maintain life. They make predictions based on these understandings. B2.2 Organic Molecules There are four major categories of organic molecules that make up living systems: carbohydrates, fats, proteins, and nucleic acids. B2.2x Proteins Protein molecules are long, usually folded chains composed mostly of amino acids and are made of C, H, O, and N. Protein molecules assemble fats and carbohydrates; they function as enzymes, structural components, and hormones. The function of each protein molecule depends on its specific sequence of amino acids and the shape of the molecule. Content Expectations: (priority expectations are in BOLD) B2.2A: Explain how carbon can join to other carbon atoms in chains and rings to form large and complex molecules. B2.2B: Recognize the six most common elements in organic molecules (C, H, N, O, P, S). B2.2C: Describe the composition of the four major categories of organic molecules (carbohydrates, lipids, proteins, and nucleic acids). B2.2D: Explain the general structure and primary functions of the major complex organic molecules that compose living organisms. B2.2E: Describe how dehydration and hydrolysis relate to organic molecules. B2.2f: Explain the role of enzymes and other proteins in biochemical functions (e.g., the protein hemoglobin carries oxygen in some organisms, digestive enzymes, and hormones). B2.4 Cell Specialization In multicellular organisms, specialized cells perform specialized functions. Organs and organ systems are composed of cells and function to serve the needs of cells for food, air, and waste removal. The way in which cells function is similar in all living organisms. B2.4f: Recognize and describe that both living and nonliving things are composed of compounds, which are themselves made up of elements joined by energy-containing bonds, such as those in ATP. B2.5 Living Organism Composition All living or once-living organisms are composed of carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates and lipids contain many carbon-hydrogen bonds that also store energy B2.5x Energy Transfer All living or once living organisms are composed of carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates and lipids contain many carbon-hydrogen bonds that also store energy. However, that energy must be transferred to ATP (adenosine triphosphate) to be usable by the cell. B2.5A: Recognize and explain that macromolecules such as lipids contain high energy bonds. Return to Unit 1 Table of Contents Instructional Background Information: What is an organic compound? All organic compounds contain carbon. The six most common elements in organic compounds are C, H, N, O, P and S. Life’s molecular diversity is based on the properties of carbon Macromolecules have thousands of covalently connected atoms. The atoms are connected by high energy bonds. What happens when you make a bond? You store energy What happens when you break a bond? You release energy Monomers are the building blocks of polymers. Dehydration synthesis (picture above) forms polymers by removing a water molecule. Hydrolysis is the reverse of dehydration synthesis. Hydrolysis (picture below) uses water to break bonds and form smaller molecules. There are four major classes of macromolecules: Carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates Monosaccharides are monomers. Disaccharides and polysaccharides are polymers. Cells link single sugars to form disaccharides Disaccharides are double sugars. Double sugars are made by the process of dehydration synthesis. Examples include maltose and sucrose: a. glucose + glucose = maltose + water monomer + monomer = polymer + water b. glucose + fructose = sucrose + water monomer + monomer = polymer + water Polysaccharides are long chains of sugar monomers. Polysaccharides are polymers of hundreds or thousands of monosaccharides linked together by dehydration synthesis. a. Plants store polysaccharides as starch in their roots. b. Animals store polysaccharides as glycogen in their liver and muscle cells. c. Plants use a polysaccharide called cellulose to build plant cell walls. Lipids Lipids include fats, which are mostly energy-storage molecules. Lipids consist mainly of carbon and hydrogen linked by non-polar covalent bonds. Lipids are not attracted to polar water molecules which make lipids hydrophobic. “Fats” a.k.a. triglycerides are 3 fatty acids attached to the 3 carbons of a glycerol. Glycerol = 3-carbon chain Fatty acids = long chains of about 15 carbons Return to Unit 1 Table of Contents Lipids are made by the process of dehydration synthesis Different kinds of fats: • Single bonds – saturated fats (usually solids from animals such as butter) • Double or triple bonds – unsaturated fats (usually liquids from plants such as corn and vegetable oils) Atherosclerosis is a condition caused by too much saturated fat in your diet causing lipid deposits (plaque) to accumulate in your blood vessels, reducing blood flow. Trans fats are unsaturated fats converted to saturated by adding hydrogens. Example: usually a softened solid such as margarine. Trans fats used to be called hydrogenated fats. Phospholipids, waxes, and steroids are lipids with a variety of functions Phospholipids - structurally similar to fats but contain phosphorus + 2 fatty acids (not 3) are used mainly in membranes of the cell. Waxes – one fatty acid linked to an alcohol (coating for fruits / insects have waxy coats to prevent dehydration). Steroids – carbons form rings such as in the case of cholesterol (also found in cell membranes). Proteins Proteins are essential to the structures and activities of life. Proteins are biological polymers constructed from amino acid monomers. There are seven major classes of proteins: 1. Structural proteins - include hair, silk of spiders, fibers of ligaments/tendons, etc. 2. Contractile proteins – the type involved in muscle movement 3. Storage proteins – ovalbumin (egg whites) for developing embryos 4. Defensive proteins – antibodies to fight infection 5. Transport proteins – hemoglobin that transports oxygen in blood stream 6. Signal proteins – hormones that serve as messengers from one cell to another 7. Enzyme proteins - serve as chemical catalysts in regulating almost all chemical activity (reactions) in the body More information about enzymes • Enzymes are large proteins that act as catalysts. What is a catalyst? Catalysts either jump start or speed up the rate of a chemical reaction. • Catalysts are recycled, they are not used up in the reaction. • Catalysts lower the amount of energy needed to turn reactants into products. • Remember: Return to Unit 1 Table of Contents Reactants Products How does an enzyme work? A specific enzyme catalyzes each cellular chemical reaction like each lock fits only one key. • Each enzyme has a part called the active site where the specific substrate binds. • The reactant is called the substrate. o In the hydrolysis of sucrose, sucrose is the substrate (reactant). o Sucrase is the name of the enzyme that catalyzes this reaction. o The enzyme allows hydrolysis (adding a water molecule) to happen. o The molecule of sucrose is then broken apart into a molecule of glucose and a molecule of fructose. o The enzyme sucrase goes on to find other sucrose molecules and repeat the process. • At the end of the reaction, the substrate changes into the product and the enzyme is released. What are proteins made of? Proteins are made from just 20 kinds of amino acids. All amino acids have the same basic structure. The R group is what varies in the 20 different kinds of amino acids. How are proteins made? Proteins are made by the process of dehydration synthesis. Nucleic Acids Nucleic acids are information-rich polymers of nucleotides Nucleic acids are the blueprints for proteins There are two types of nucleic acids: DNA – deoxyribonucleic acid RNA – ribonucleic acid Genetic material consists of DNA, and within the DNA are gene. Genes are specific stretches of the molecule that program the amino acid sequence of proteins. Monomers that make up nucleic acids are called nucleotides. RNA is a single polynucleotide strand and DNA is a double polynucleotide strand that twists to form a double helix. Return to Unit 1 Table of Contents Pictures in Instructional Background Information: Campbell, Neil. (2000) Biology: Concepts and Connections. San Francisco, Addison Wesley Longman, Inc. Return to Unit 1 Table of Contents Terms and Concepts ATP Carbohydrate Catalyst Chemical bond Covalent bonds DNA Dehydration synthesis Element Enzyme Hemoglobin High energy bonds Hormone Hydrolysis Lipid Nucleic acid Protein Polymers RNA Substrate Knowledge and Skills Student should be able to: Identify representations of large biological molecules as polymers of simple subunits. Identify structural formula of amino acids. Identify the structural relationships formulas of monomers of fats, proteins, carbohydrates (fatty acids, amino acids, simple sugars). Identify the between enzymes and substrates. Identify the role of dehydration synthesis and hydrolysis in the building and breaking down of macromolecules. Return to Unit 1 Table of Contents Instructional Resources: This website has many hands on biology activities. http://serendip.brynmawr.edu/sci_edu/waldron This website has links to biology labs and lectures. www.explorebiology.com This website has great interactive simulations. http://www.explorelearning.com/ Return to Unit 1 Table of Contents Chemistry and Biochemistry Activity #1: Who took Jerell’s iPod? Questions to be investigated: What are the different types of organic compounds? How are these types of compounds tested for? Biology HSCE’s addressed: B2.2 Organic Molecules There are four major categories of organic molecules that make up living systems: carbohydrates, fats, proteins, and nucleic acids. B2.2x Proteins Protein molecules are long, usually folded chains composed mostly of amino acids and are made of C, H, O, and N. Protein molecules assemble fats and carbohydrates; they function as enzymes, structural components, and hormones. The function of each protein molecule depends on its specific sequence of amino acids and the shape of the molecule. B2.2C: Describe the composition of the four major categories of organic molecules (carbohydrates, lipids, proteins, and nucleic acids). Materials: -Containers for testing food such as test tubes, specimen jars, etc. -Stirrers, such as plastic spoons -Masking tape for labeling testing containers -Biuret reagent for protein testing (approximately 4 ml per student lab group) -Iodine-Potassium Iodide Solution for starch testing (approximately 1 ml per student lab group) -Glucose test strips (5 per student lab group for the first day and 1 per student lab group for the second day) – an alternative to the strips is Benedict’s solution -Brown paper bag for lipid testing (1 per student lab group; half for each day) -Gloves (1 or 2 per student for each day) -Samples for testing Day 1: (approximately 1.5 ml of each per student lab group) Vegetable oil Corn starch Powdered egg whites (can be found in the baking needs aisle) Glucose (may also be sold as Dextrose, can be found online, in the pharmacy often times in tablet form, or sometimes, in a cake decorating supply store (e.g. Joann’s)) Day 2: (approximately 3 ml of each per class) Pretzels Butter Jelly (You may want to make sure this tests positive for glucose; we have had success with strawberry jelly and we believe that any jelly sweetened with high fructose corn syrup will test positive for glucose.) Fat-Free or low-fat vanilla or plain yogurt Beans (canned beans that have been mashed into a paste; e.g. canned white beans) *Save the labels with nutrition information from all the food packages. These will be useful for discussing any discrepancies between predictions and observed results. Return to Unit 1 Table of Contents Return to Unit 1 Table of Contents Safety Concerns: Students should at least wear gloves while performing tests for carbohydrate and proteins; goggles are also recommended. You may also want to keep the Biuret reagent and iodine solution at your desk and have students come to pick it up when they need it. Real-World Connections: Students relate study of macromolecules to the nutrients in the food they eat. Teacher Notes: This activity reinforces student understanding of different types of organic compounds and several aspects of scientific method. Before you begin this activity, your students should be familiar with the basic chemical structures and general properties of carbohydrates, proteins and lipids. Helpful teacher notes can be found online at http://serendip.brynmawr.edu/exchange/waldron/organic You will need to make evidence samples for Day 2. We suggest that you make four different pairs of dry and liquid evidence samples so different student groups will get different results. Worker in break room Jose Ashley Bruce Kiara Lunch/Snack Bean burrito with cheese Fat-free yogurt Toast with butter and jelly Pretzels Solid Evidence Glucose Liquid Evidence Lipid A - + + A + (oil) B + - + B - (water) C + + - A + (oil) D - + - B - (water) Starch Protein Procedure/Description of Lesson: In the first class period, students learn how to use chemical indicators to test for different types of organic compounds, and in the second class period each student group will test one or two types of food or a sample of evidence to figure out who took Jerell's iPod. You may want to assign each type of food or evidence to two student groups in order to assess reliability of results. Source: Dr. Jennifer Doherty, Dr. Ingrid Waldron and Dr. Lori Spindler, Department of Biology,University of Pennsylvania, copyright 2009 1, serendip.com, Adapted from “Identity of Organic Compounds” from Biology Laboratory Manual A from Prentice-Hall; Also inspired by “Crime Scene Activity” by Kathy Paris, Bethel High School Who took Jerell’s iPod? -- An organic compound mystery Jerell is a 10th grade student at City High School who works at McDonald’s on the weekends. While on break, Jerell was studying for his biology test and listening to his new iPod. There were four other workers taking a break at the same time, each having something different for lunch. Jerell’s girlfriend stopped by near the end of his break, and he rushed out to see her and forgot his iPod and biology book in the break room. When he realized he had forgotten it, he hurried back and found only his biology book and some food crumbs. His iPod was gone! Return to Unit 1 Table of Contents Return to Unit 1 Table of Contents First, Jerell freaked out, but he calmed down when he realized he could use his knowledge of organic compounds to figure out which of his coworkers left the crumbs on his textbook and who took his iPod. What are organic compounds? Almost all of the food we eat comes from plants and animals. Plants and animals contain mainly water and organic compounds, which are molecules made by living organisms such as plants or animals. The table below lists the most common types of organic compounds found in living organisms. For each type of organic compound, give one or two examples and describe one characteristic, e.g. whether it is greasy, whether it contains genetic material, whether there is lots of this type of organic compound in meat or lots in pretzels and potatoes. DATA TABLE 1: Type of Organic Examples of Food That Has Characteristic of Food That Compound Lots of This Type of Organic Has Lots of This Type of Compound Organic Compound Carbohydrates Lipids Nucleic acids Proteins Today you will be testing the substances listed in the following table. Predict whether each substance is an organic compound and if so, what type. DATA TABLE 2: Substance Do you think this substance is a carbohydrate, lipid, protein, or none of these? Vegetable oil Glucose Starch from corn or potatoes Powdered egg whites Water What are indicators? An indicator is a substance that changes color in the presence of a particular type of molecule. Today you will learn how to use several indicators to test for the presence of carbohydrates and proteins. You will also use a different type of test for lipids. Tomorrow, you will use these tests to analyze several types of food and the evidence left at the scene of the crime to find out who left the crumbs on Jerell’s textbook. Testing for lipids 1. If a food that contains lipids is put on brown paper, it will leave a spot that lets light through. To test for lipids, divide a piece of a brown paper bag into 5 sections. Label the sections "vegetable oil", "glucose", "starch", "egg whites", and “water”. 2. In each section, rub a small amount of the substance onto the brown paper. Return to Unit 1 Table of Contents Return to Unit 1 Table of Contents With a paper towel, rub off any excess that may stick to the paper. Set the paper aside until the spots appear dry—about 10 to 15 minutes. 3. Which section do you expect to test positive for lipids? 4. Which sections do you expect to test negative for lipids? 5. Continue on with the rest of the tests. After all the sections of the brown paper are dry, hold it up to a bright light or window. You will notice that at least one sample has left a spot that lets light through on the brown paper. The spot indicates the presence of lipids. 6. Complete the last column of data table 3. Put a plus for any samples which tested positive for lipids and a minus for the samples which tested negative. DATA TABLE 3: Carbohydrate Tests Sample Test strip color Glucose present Iodine test color Protein Test Starch present Biuret test color Protein present Lipid Test Lipid present Vegetable oil Glucose Starch from corn or potatoes Powdered egg whites Water Testing for Carbohydrates 1. You must wear gloves to protect yourself. 2. You will use indicators to test for two common types of carbohydrates: glucose (a specific type of sugar) and starch. Obtain 5 containers and use masking tape to make labels for each container. Label the containers "vegetable oil", "glucose", "starch", "egg whites", and “water”. 3. For each container, add a small amount of the substance indicated on the masking-tape label. Now add about 2 ml of water to each container. Stir the contents of each container to mix the sample and water. Return to Unit 1 Table of Contents Return to Unit 1 Table of Contents To test for glucose you will use a test strip with an indicator pad that changes color in the presence of glucose. Prepare a piece of paper with the name of each substance and a place to put the glucose test strip used to test that substance. Dip one test strip into each sample for 1-2 seconds. Remove the strip, put it in the appropriate spot on your labeled paper, and wait 3 minutes. 4. Which substance do you expect to test positive for glucose? 5. Which substances do you expect to test negative for glucose? 6. After 3 minutes, record the color for each glucose test strip in the data table 3. Put a plus next to those samples testing positive for glucose and a minus for those testing negative. 7. To test for starch you will use iodine as an indicator. In the presence of starch, iodine will change color from yellow-brown to blue-black. Add 5 drops of iodine solution to each container. Stir the contents of each container. CAUTION: Be careful when handling iodine; it can stain hands and clothing. 8. In data table 3, record the color of the iodine solutions. Put a plus next to those samples testing positive for starch and a minus for those testing negative. Testing for Proteins 1. Label five clean containers "vegetable oil", "glucose", "starch", "egg whites", and “water”. Add a small amount of the substance indicated on the label to each container. Add about 2 ml of water to each container. Stir the contents of each container to mix the food and water. 2. To test for protein you will use Biuret reagent as an indicator. Biuret reagent turns from blue to purple in the presence of protein. Add 20 drops of biuret reagent to each container. Stir the contents of each container. CAUTION: Biuret reagent contains sodium hydroxide, a strong base. Be very careful not to splash or spill any. If you splash any reagent on yourself, wash it off immediately with water. Call your teacher for assistance. 3. Record the color of each Biuret solution in data table 3. Put a plus next to those samples testing positive for protein and a minus for those testing negative. 4. Rinse all ten containers thoroughly. Questions 1. Compare your predictions from data table 2 with your test results in data table 3. Were there any differences between your test results and your predictions for what type of organic compound each test substance is? Return to Unit 1 Table of Contents Return to Unit 1 Table of Contents If you found any differences between your predictions and your results, what do you think is the reason for these differences? You may want to check with your teacher, your textbook, or the nutrition information in the label on each food package to help you interpret your results. Did your test for glucose indicate there was glucose in the starch sample? Does that mean that there is no glucose in starch? (Hint: Check your textbook or other reliable source if you do not already know the chemical structure of starch.) This result shows that the glucose indicator is quite specific. It reacts with glucose dissolved in water, but it does not react with glucose molecules that are combined into a large organic compound like starch. 2. Suppose that for the container containing water you found a positive test for one of the organic compounds. How would you interpret this result? Testing Different Types of Food and Testing the Evidence Today you will perform all four organic compound tests on one or two of the types of food listed below or on the evidence that Jerell found at the crime scene (your teacher will assign you samples to test). Begin by predicting which types of compounds you expect to find in each type of food you will be testing. DATA TABLE 4 Food Do you expect this food to contain Glucose? Starch? Protein? Lipid? Pretzel Butter Jelly Fat-free yogurt Beans Record your positive and negative test results using plus and minus signs in the data table below. The evidence that Jerell found has been separated into a liquid and a solid in two separate bottles. After you perform the tests, your teacher will collect your data to share with the rest of the class. Complete the table below using data from your classmates. DATA TABLE 5 Lipid Carbohydrate Tests Protein Test Test Food Test strip Glucose Iodine Starch Biuret Protein Lipid color present test color present test color present present Pretzel (crumble into the container) Butter Jelly Fat-free yogurt Beans (mash into a paste) Return to Unit 1 Table of Contents Dry part of Jerell’s evidence Liquid part of Jerell’s evidence Who took Jerell’s iPod? The workers in the break room are listed below with the type of lunch they were eating while Jerell was studying. As preparation for interpreting the evidence, complete the chart below to indicate what kinds of organic compounds are found in each type of food and what kinds of organic compounds were found in the combined liquid + dry evidence. Worker in break Lunch/Snack Glucose Starch Protein Lipid room Jose Bean burrito with cheese Ashley Fat-Free Yogurt Bruce Toast with butter and jelly Kiara Pretzel Thief Combined liquid + dry evidence Complete the following table to summarize the evidence and your interpretation of the evidence. Did he/she Worker take How do you know? in break Jerell’s Describe the evidence that supports your conclusion. room ipod? Jose Ashley Bruce Kiara Return to Unit 1 Table of Contents Analysis Questions: 1. Who took Jerell’s iPod? Do you have any doubts about your conclusion? Explain. 2. Our bodies are made up of the same types of organic compounds as all other living organisms. Complete the following sentences by filling in each blank to indicate the function of each type of molecule in different parts of our body. Our muscles contain lots of protein. This protein enables the muscles to _____________. Glucose is carried by our blood to all the cells in our body. Our cells use the glucose for _______________. Lipids are found in fat cells in our bodies. The fat cells store fat molecules to be used for ______________ if a person cannot get enough food. Our bodies do not make starch, but we often eat plant foods which contain starch which we digest to _____________, the building block that is used to make starch. DNA is a nucleic acid that is found in every cell. DNA carries the ____________ information. 3. To show your understanding of organic compounds, identify the type of organic compound shown in each diagram and complete the first three columns of the table. 4. Many large organic compounds are made of multiple repeats of smaller building block compounds. Starch, proteins, and nucleic acids are examples of this type of organic compound. Circle a building block in the starch, protein, and nucleic acid figures, and write the name of the building block in the fourth column. Which test is used to Type of Name of Diagram of Structure of Organic detect this compound Functions Organic building Compound or type of Compound block compound? Return to Unit 1 Table of Contents Not tested for Return to Unit 1 Table of Contents Return to Unit 1 Table of Contents Chemistry and Biochemistry Activity #2: Analyzing Food Labels Questions to be investigated: What types of organic compounds are in the food we eat? Is high fructose corn syrup worse for you than sugar? Biology HSCE’s addressed: B2.2C: Describe the composition of the four major categories of organic molecules (carbohydrates, lipids, proteins, and nucleic acids). Materials: Food Labels Safety Concerns: N/A Real-World Connections: Students relate study of macromolecules to the nutrients in the food they eat by studying sugar and HFCS. Teacher Notes:. Students collect food labels from home showing products both with and without hfcs. You can introduce this activity by showing one of the commercials discussing high fructose corn syrup. Student understanding can be assessed through by having students conduct research on the opposing viewpoints of high fructose corn syrup and conduct a debate in class. Procedure/Description of Lesson: Students will find food labels at home that have different sweeteners. They will then research the different sweeteners and have a debate. Instructions: 1. Looking in your pantry or cupboards at home, read the labels to find out which foods contain high fructose corn syrup, glucose (also known as dextrose), or no high fructose corn syrup. 2. Staple or tape each label in the appropriate space below. You must highlight or underline the label where the type and amount of the sugar is identified. High Fructose Corn Syrup (HFCS) label including the ingredients: Staple label here and highlight or underline high fructose corn syrup (HFCS)! Glucose (also known as dextrose) label including the ingredients: Return to Unit 1 Table of Contents Return to Unit 1 Table of Contents Staple label here and highlight or underline glucose/dextrose! A label with NO High Fructose Corn Syrup (HFCS): Staple label here that has NO high fructose corn syrup (HFCS)! Assessment Ideas: Student understanding can be assessed through class discussion at the conclusion of the activity as well as completion of the assignment and article extension. Also, you can follow up this activity by having students conduct research on the pros/cons of high fructose corn syrup and conduct a debate in class. The debate could be done as a ‘silent debate.’ This is a writing strategy where each student must pick a side. The first student writes down a statement to support their position, passes the paper to the second student who debates that statement with their own argument. This continues for a set amount of time (~7 minutes). Opposing viewpoints can be found at the following links: McLaughlin, Lisa. “Is High-Fructose Corn Syrup Really Good for You?” Time.com. September 17, 2008 Zeratsky, Katherine. “High-fructose corn syrup seems to be a common ingredient in many foods. What are the concerns about high-fructose corn syrup?” Mayoclinic.com Princeton University. "High-Fructose Corn Syrup Prompts Considerably More Weight Gain, Researchers Find." ScienceDaily 22 March 2010. 25 June 2010 <http://www.sciencedaily.com /releases/2010/03/100322121115.htm Return to Unit 1 Table of Contents Return to Unit 1 Table of Contents Chemistry and Biochemistry Activity #3: Pineapple Enzymes and Protein Lab Questions to be investigated: How do enzymes and substrates work? Biology HSCE’s addressed: B2.2f: Explain the role of enzymes and other proteins in biochemical functions (e.g., the protein hemoglobin carries oxygen in some organisms, digestive enzymes, and hormones). Materials: Fresh pineapple Boiling & ice water Canned pineapple Test tubes & rack Frozen pineapple Spoons, stirring rods Jell-O Knife for chopping pineapple Beakers Safety Concerns: Goggles are recommended. Real-World Connections: Students relate study of macromolecules to the nutrients in the food they eat by studying sugar and HFCS. Teacher Notes: Students will need to be familiar with the scientific method and have an understanding of macromolecules. Gelatin is made from the protein collagen (from the joints of animals). To make Jell-O, gelatin is dissolved in hot water, then allowed to cool. When the dissolved gelatin cools, collagen makes a matrix, trapping the water. This is what results in the jiggly texture of Jell-O. Bromelain(found in fresh pineapple) breaks down collagen, this enzyme is denatured in canned pineapple. In this lab, pineapple contains the enzyme bromelain, jello is the substrate and the processing of pineapple denatures the enzyme bromeain. Procedure/Description of Lesson: Students will create a lab to study the affect of pineapple on gelatin. Source: adapted from a lab by Kim Foglia, explorebiology.com Background information: If you have ever made Jell-O by cooking the powder that comes in a box, you may have noticed the warning on the instructions that tell you not to add fresh or frozen pineapple to the gelatin. Have you ever wondered why? In this lab, you will be designing an experiment to test what is really happening when you add pineapple to gelatin. You know enough organic chemistry now to figure this out. First, you need a little background about gelatin… and it may be more than you ever wanted to know. Do you know what Jell-O is really made out of? Are you ready? That sweet colorful treat is actually made out of hides, bones, and inedible connecting tissue from animals butchered for meat. No? Yup! All gelatin (including those made for photographic and laboratory use, as well as for desserts) is made out of discarded animal parts — the tough parts: bone and skin. And all these tough parts are made of proteins. In fact, the extracted gelatin is a protein. So, why do you think gelatin gets thick and jelly-like when you cook it? (We’ll come back to that later.) Gelatin can be extracted from any kind of animal, but cows are most common. If your Mom or Dad have ever made a batch of chicken soup from scratch, you've probably seen how it gets stiff and Jell-O like after it sits in the fridge… that's because boiling the chicken in water extracts the gelatin from the carcass (bones & cartilage), just like a miniature version of the commercial gelatin factories! Commercial gelatin making starts by grinding up bones. The crushed bones are then soaked in a strong base (high pH) to soften them, and then passed through Return to Unit 1 Table of Contents progressively stronger acid (low pH) solutions, until the end result isn't recognizable as bones at Return to Unit 1 Table of Contents all! Then the whole mess is boiled for hours to extract the gelatin… and this part really makes a stink! Finally, the gelatin layer is skimmed off the boiling pot, and dried into a powder. With added sugar, flavorings, and artificial color, it's ready to become a jiggly dessert! And now that you know what Jell-O's made from, why don't you put some on the table tonight? Your guests will be delighted when you share your new knowledge with them in the middle of a luscious spoonful of dessert! By the way, this whole process of extracting gelatin from bone was originally developed in 1845 by an engineer, Peter Cooper — the man who Cooper Union (in NYC) is named after. Sometime later (1895), Pearl B. Wait, a cough syrup manufacturer, bought the patent from Peter Cooper and adapted Cooper's gelatin dessert into an entirely prepackaged form, which his wife, May David Wait, named "Jell-O." The rest is history... Made from bone… made from protein… so it must be tough stuff! So why can’t you put fresh pineapple in it? Let’s learn a bit about pineapple. The pineapple plant (Ananas comosus) is a monocot, or grass-like plant, that belongs to the bromeliad family. It is thought to have originated in Brazil. In the 1950s, pineapple became the United State’s second most important fruit and Hawaii led the world in both quantity and quality of pineapples. However, times have changed and now, all canned pineapple comes from overseas, largely from the Philippines. As with some other tropical fruits, the pineapple fruit contains an enzyme that breaks down, or digests, protein. This protease (protein-digesting) enzyme in pineapple is called bromelain, which is extracted and sold in such products as Schilling's Meat Tenderizer. Papaya, another tropical fruit, also contains an enzyme, called papain, that digests protein. It can be found in Accent Meat Tenderizer. Procedure: In this lab, you will be given an array of materials and you will be asked to design your own experiment to test the effect of pineapple on gelatin. The goal is to understand what is actually going on in the pineapple-gelatin mix at a chemical level as well as understanding what affects the function of enzymes. Design a controlled experiment that shows the effect of raw pineapple on gelatin. Make sure your experiment description includes the following: A hypothesis A detailed experimental design which will include: The effect of fresh pineapple on gelatin. The effect of frozen pineapple on gelatin. The effect of canned pineapple on gelatin. The effect of freshly cooked pineapple on gelatin. A data table You will be able to perform your experiment once you receive approval of your experimental design from your teacher. Return to Unit 1 Table of Contents Return to Unit 1 Table of Contents Experimental Design Guide Teacher Approval_________ Title: Hypothesis: Independent Variable: Measurement of Independent Variable: Number of Trials: Dependent Variable: Measurement of Dependent Variable: Control: Other Controlled Factors (At Least 5): Questions: 1. Clearly describe the results of your experiment. In which test tubes did the gelatin jell, which did not. 2. Clearly explain the results of your experiment. Why did some test tubes of gelatin jell, why did others not. Be specific! 3. What is the enzyme in your experiment? 4. What is the substrate in your experiment? 5. What is (are) the product(s) in your experiment? 6. What type of organic molecule is gelatin? 7. What type of organic molecule is bromelain? 8. Write a “word equation” to describe the chemical reaction that occurs when pineapple is mixed with the gelatin. 9. Is the reaction of bromelain and gelatin dehydration synthesis or hydrolysis? Explain. 10. Why were the results of the freshly cooked pineapple different than the results of the fresh, raw pineapple? Be specific! 11. What is meat tenderizer and what does it do? Assessment Ideas: On the accompanying sheet of paper, design an experiment to test at what specific temperature the pineapple enzyme denatures. Title: Hypothesis: Independent Variable: Measurement of Independent Variable: Number of Trials: Dependent Variable: Measurement of Dependent Variable: Control: Other Controlled Factors (At Least 5): Return to Unit 1 Table of Contents Return to Unit 1 Table of Contents Chemistry and Biochemistry Activity #4: Dehydration Synthesis and Hydrolysis Virtual Simulation Questions to be investigated: How do the processes of dehydration synthesis and hydrolysis work to build and break organic molecules? Biology HSCE’s addressed: B2.2E: Describe how dehydration and hydrolysis relate to organic molecules. Materials: Computers with internet access Safety Concerns: N/A Real-World Connections: Students will use a virtual simulation to visualize dehydration synthesis and hydrolysis. Teacher Notes: This lesson would fit best after students have obtained a solid understanding of macromolecule structure and dehydration synthesis and hydrolysis. The simulation is found on the site ‘explorelearning.com.’ The name of the ‘gizmo’ is ‘dehydration synthesis.’ If your school does not have access to this website, you can sign up for a free 30 day trial. Procedure/Description of Lesson: Students build on prior knowledge and view simulation of dehydration synthesis and hydrolysis. Source: ExploreLearning Gizmos™ Dehydration Synthesis Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. If you exercise on a hot day, you need to worry about dehydration. In this context, what do you think dehydration means? 2. Astronauts and backpackers often bring dehydrated food. What do you think dehydrated food is? Gizmo Warm-up What do rice, potatoes, and sugar have in common? They are all foods rich in carbohydrates. Carbohydrates are an important energy source for your body. The basic building block of most carbohydrate compounds is the molecule glucose. Using the Dehydration Synthesis Gizmo™, you will learn about the structure of a glucose molecule and how glucose molecules can be joined together to make larger carbohydrate molecules. To begin, select the CREATE GLUCOSE tab. 1. Look at the chemical formula for glucose. How many carbon (C), hydrogen (H), and oxygen (O) atoms are found in a molecule of glucose? C:_______ H:_______ O:_______ 2. Turn on Show chemical structure. Each black sphere represents a carbon, hydrogen, or oxygen atom. The lines connecting the spheres represent chemical bonds. How many black spheres are in the diagram? _______ Return to Unit 1 Table of Contents Return to Unit 1 Table of Contents A. How does this relate to the number of carbon, hydrogen, and oxygen atoms in the chemical formula for glucose? ___________________________________________ Activity A: Get the Gizmo ready: Be sure the CREATE GLUCOSE tab is still Build a glucose selected. molecule Introduction: Each element tends to form a certain number of chemical bonds. This value is the valence of the element. For example, a carbon atom has a valence of four. Goal: Construct a molecule of glucose. 1. Identify: The structure of a water molecule (H2O) can be written as H-O-H, which each dash representing a chemical bond. Count the number of bonds the oxygen and hydrogen atoms form in a water molecule. A. What is the valence of oxygen? _______ _______ 2. B. What is the valence of hydrogen? Build a model: Use the carbon, oxygen, and hydrogen atoms from the Atoms box to build a glucose molecule on the empty hexagon in the building region. Use the chemical structure in the lower right as a guide, and pay attention to the valence of each atom as you build. Once you think you have correctly constructed the glucose molecule, click Check. If necessary, continue to modify your molecule until it is correct. 3. Make a diagram: Congratulations, you have completed a molecule of glucose! Click the COPY SCREEN button to take a snapshot of your completed molecule. Paste the image into a blank document and label the image “Glucose.” 4. Explain: How did the valence of each element help you determine the structure of the glucose molecule? 5. Make connections: Carbon forms the backbone of every major type of biological molecule, including carbohydrates, fats, proteins, and nucleic acids. How does carbon’s high valence relate to its ability to form these large and complex biomolecules? Activity B: Dehydration synthesis Get the Gizmo ready: • Select the DEHYDRATION tab. Return to Unit 1 Table of Contents Return to Unit 1 Table of Contents Question: What occurs when two glucose molecules bond? 1. Infer: Glucose is an example of a monosaccharide, the simplest type of carbohydrate. A disaccharide is made from bonding two monosaccharides together. A. What do you think the prefixes mono- and di- mean? Mono-: __________ Di-: __________ 2. Predict: Turn on Show description. Drag both glucose molecules into the building region. Observe the highlighted region. What do you think will happen to the atoms in this region when the glucose molecules bond? 3. Run Gizmo: Click Continue and watch the animation. A.What happened? B.What was removed from the glucose molecules when they bonded to form maltose? 4. Infer: Based on what you have seen, create a balanced equation for the dehydration synthesis reaction. (Recall that the formula for glucose is C6H12O6.) You will have to determine the formula of maltose yourself. 5.Turn on Show current formula/equation to check your answer. 6.Summarize: Use what you have observed to explain what occurs during a dehydration synthesis reaction. 7.Apply: A trisaccharide is a carbohydrate made of three monosaccharides. What do you think would be the chemical formula of a trisaccharide made of three bonded glucose molecules? Get the Gizmo ready: Activity C: • Select the Hydrolysis tab. • Turn on Show description and Show current Hydrolysis formula/equation. Introduction: Carbohydrates made up of three or more bonded monosaccharides are known as polysaccharides. In a reaction known as hydrolysis, your body breaks down polysaccharides into individual monosaccharides that can be used by your cells for energy. 1. Predict: Examine the polysaccharide in the building region and its chemical formula. A. How many monosaccharides can form if this polysaccharide breaks up? B. Recall the formula of glucose is C6H12O6. How many carbon, oxygen, and hydrogen atoms will you need for three glucose molecules? Return to Unit 1 Table of Contents C. What must be added to the polysaccharide in the Gizmo to get three glucose molecules? 2. Observe: Turn off Show current formula/equation. Drag a water molecule into the building region. Click Continue. What happened? 3. Infer: Create a balanced equation for the hydrolysis reaction that just occurred. Turn on Show current formula/equation to check your answer. 4. Observe: Turn off Show current formula/equation. Drag the second water molecule into the building region. Click Continue. What happened? 5. Summarize: Now create a balanced equation that shows the entire hydrolysis reaction. (In other words, the equation should show how the polysaccharide broke up into three separate glucose molecules.) Turn on Show current formula/equation to check your answer. 6. Compare: How do hydrolysis reactions compare to dehydration synthesis reactions? 7. Apply: Amylose is a polysaccharide made from the synthesis of four glucose molecules. A. How many water molecules are produced when amylose forms? B. What do you think is the chemical formula for amylose? C. How many water molecules would be needed to break amylose down into four glucose molecules? 8. Extend your thinking: Hydrolysis of the carbohydrates you eat begins in your mouth as you chew. How do you think this process might be affected if a person’s salivary glands were unable to produce saliva, which is mostly composed of water? Assessment Ideas: Gum Drop Chemistry Have students use their diagrams of a glucose molecule from activity A of the Student Exploration sheet to build a model of glucose using toothpicks and gum drops (or mini marshmallows). Make sure all students use the same color of gum drops to represent each type of atom. Write the color key on the board as a reminder for students. After each student builds their gum drop model, have students form pairs. The students should model how two glucose molecules can bond to form a molecule of maltose and a molecule of water. Next, have students model hydrolysis by reversing the reaction. This can also be done with molecular kits. Return to Unit 1 Table of Contents