* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Grazing incident X-ray Diffraction
Double-slit experiment wikipedia , lookup
Photoelectric effect wikipedia , lookup
Introduction to quantum mechanics wikipedia , lookup
Cross section (physics) wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Monte Carlo methods for electron transport wikipedia , lookup
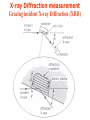
Grazing incident X-ray Diffraction (XRD)
X-rays are electromagnetic radiation with very short wavelength ( 10-8 10-12 m), very suitable to do diffraction for the crystal. For photon E = h
= hc/, 5000 eV photon has wavelength of 2.5 Å. XRD is a well-known
technique to study bulk crystal structure.
Bragg’s Law: n= 2dsin()
X-ray Diffractometer
Detector
X-ray Tube
Sample stage
X-ray Diffraction measurement
Grazing incident X-ray Diffraction (XRD)
Typical
spectra
Besides the angle
distribution,
the
intensity and width of
the peak can also
gives
information
about the sample, such
as particle or grain
size, and strain, etc.
Grazing incident X-ray Diffraction (XRD)
As x-ray generally have rather large penetration depth than electrons,
it is far from surface sensitive. The solution for study of the surface
structure is to use grazing angle (1-3O).
X-ray photoelectron diffraction (XPD)
Forward scattering dominates the
diffraction of the electrons with
kinetic energy over several
hundreds of eV.
Forward scattering
Strong intensity peak along low index
axis of the crystal due to forward
scattering at hundreds eV. This can be
used to do element-specific (XPS)
structure analysis with very high
surface sensitivity.
An application
For Co film grown on fcc Cu
substrate. Fcc structure have
inplane lattice distance same as
out-of-plane one, the (011) axis
is 45 degree with respect to the
sample normal. For Fct (fcc
distorted) these two parameters
are different, (011) is along
some other angle.
Magnetic structural
information from dichroism
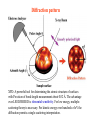
Diffraction pattern
XPD: A powerful tool for determining the atomic structure of surfaces
with Precision of bond-length measurement about 0.02 Å. The advantage
over LEED/RHEED is elemental sensitivity. For low energy, multiplescattering theory is necessary. For kinetic energy over hundreds of eV the
diffraction permits a single-scattering interpretation.
Other particles scattering
Atoms/Molecules such as He
atom can be scattered from
surface, they can be used to
study surface structures with
very high surface sensitivity.
Thermal
Helium
Atom
Scattering is most widely used
one. The atoms have energy of
10meV - 100meV which have
of the order of Å.
Neutron can be used to study surface
too, although neutron scattering have
very large free path length and is
bulk-sensitive. In some case small
angle scattering is useful and it has
advantage to study the interfaces.
XANES and EXAFS
X-ray Absorption
Near Edge
Structure
and
Extended X-ray
Absorption Fine
Structure
Also
elemental
sensitivity
Extended X-ray Absorption Fine Structure
(EXAFS)
Outgoing
photoelectrons
X-ray absorption decrease with increasing photon energy except at
absorption edges. After each absorption edge, the absorption undergoes
oscillations due to the interference between outgoing electron wave
(photoelectron) and part of outgoing electron wave scattered back by
neighboring atoms.
Some theoretical consideration
The absorption coefficient u can be written as: m = m0K(1+c) + m0. The
interference of outgoing wave and backscattered waves by the neighbors
leads to:
c(k) = S Ai(k) sin[2kRi + ri(k)]
i
Here k is determined by the photon energy and the binding energy of
the absorption edge. ri(k) is the scattering phase shift.
For simple case, after subtraction of the background due to m0K, one
can determined Ri from the oscillation period DE, with
Ri = (151/DE)1/2 Å
A better way is to do Fourier-transform as:
Subtraction of background
Calculate distance
-does not occur for isolated atoms
- outgoing photoelectron (KE > 50
eV) behaves like free electron
- most interference from nearest
neighbors
- sensitive to short-range order (unlike diffraction) - works for
amorphous, polycrystalline, glassy materials, liquids
- can select absorption edge for one particular species (C, O, N… )
Example
Different
detection
methods have different
surface sensitivity, for
example change of the
incident angle or choose
different signal resources.
There
is
therefore
Surface Extended Xray Absorption Fine
Structure (SEXAFS).
C K-edge NEXAFS of polymer film
3.0
The change of peak
height ratio of p* and
s* core excitations as
the change of
incident angle of the
light reveals the
alignment of polymer
molecules are
perpendicular to the
surface!
XAS (a. u.)
2.5
2.0
1.5
Incidence angle
0
50
60
normal Au signal
1.0
0.5
280
290
300
Photon energy (eV)
310
320
Rutherford BackScattering (RBS):
Conservation laws for atomic collision
a)
Information for
atoms on the
surface, elemental
sensitive!
For particles with high enough energy, their wavelength are much short
than the crystal lattice parameter and the scattering on the surface can be
treated as classic collision between the incident particles and the atoms on
the surface. The requiring of the conservation of both momentum and
energy gives the ratio between initial energy and final energy for incident
particles:
E1’/E1 = {[(m22-m12sin)1/2+m1cos]/(m1+m2)}2
Rutherford BackScattering (RBS):
scattering cross section
Considering a Coulomb field and recoil of the m2, one get
One can write in a power series when m1 smaller than m2:
Channeling and Blocking
The shadow cone exists behind
the scatterer. For particular low
incident ion, can be broad as half
a typical interatomic distance,
with increasing energy, the
shadow cone becomes smaller.
Incident ions are
steering through
the
channels
formed by the
rows of atoms
(low index axis)
with very large
penetration
length.
Angulardependent RBS
The direction of the
incident
ions
with
respect to the crystal
orientation determines
the geometry for the
scattering. Along low
index axis, channeling
happens and scattering
only at the first several
layers,
for
random
direction scattering for
all the atoms.
The applications
a) First layer atoms scatter;
b) First and second layer
atoms scatter;
c) At certain angle first and
second layer atoms scatter,
while along normal only
first layer atoms scatter;
d) Adsorbate overlayers’ first
layer atoms scatter, the first
layer atoms of substrate
scattering reduced.
e) Amorphous
overlayer
atoms scatter, there is no
peak but a bump and the
size of the bump depends
on thickness
of the
overlayer.
The applications
Thickness infor.
Angular dependence
Si with As
doped have
same structure
Yb is at
interstitial
positions
FIELD ION MICROSCOPY
smaller
than
100
nm
It consists of a sharp needle emitter and a fluorescent screen. The field
strength at the tip surface approaches 109 V/cm. With a gas of neutral atoms
of about 10-3 torr (Ne, He, H2 and Ar), gas atoms collide with tip will be
ionized after many collisions, the ions then will be accelerated away to the
screen to generate patterns. As ionization happens where the electric field is
high (protruding atoms), the spots on the screen corresponds to the position
of atoms on the tip. Resolution can reach 2.5 Å.
FIM and FEM
FIM gives atomic
image of the surface,
FEM provide current
density variation
emitted form the
surface (difference in
work functions and
electric field on the
emitter surface).
Unlike LEED, FIM
is more information
about 3D atomic
arrangement,
however, suitable to
study surface
migration across
boundaries and also
surface absorbate.