* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Chemical Earth (8.2.3)
Survey
Document related concepts
Ionic liquid wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Ionic compound wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Gibbs paradox wikipedia , lookup
Bose–Einstein condensate wikipedia , lookup
Aromaticity wikipedia , lookup
Particle-size distribution wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Homoaromaticity wikipedia , lookup
Degenerate matter wikipedia , lookup
Atomic orbital wikipedia , lookup
Heat transfer physics wikipedia , lookup
State of matter wikipedia , lookup
Electron scattering wikipedia , lookup
Microplasma wikipedia , lookup
Transcript
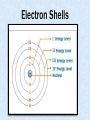
The Chemical Earth (8.2.3) Elements in Earth materials are present mostly as compounds because of interactions at the atomic level Answers to 8.2.2 Element Outer shell electrons No. Shells Symbol Atomic Number Atomic Mass Metal, semimetal non-metal Sulfur 6 3 S 16 32.1 NM Rubidium 1 5 Rb 37 85.5 M Selenium 6 4 Se 34 79 NM Boron 3 2 B 5 10.8 SM Arsenic 5 4 As 34 74.9 NM Barium 2 6 Ba 56 137.3 M Krypton 8 4 Kr 36 83.8 NM Germanium 4 4 Ge 32 72.6 SM Bromine 7 4 Br 35 79.9 NM Answers to 8.2.2 (contd.) Element Uses of element Chlorine Sterilization of water. Sodium Heat transfer fluid in nuclear power plants. Tungsten Filaments in light globes, etc. Gold Jewellery, etc. Mercury Electrical switches, etc. Zinc Protecting iron (galvanizing). Iron Major building material. Copper Copper electrical wire. Argon Inert gas used in light bulbs and welding. Helium Carry weather balloons aloft. Magnesium Structural metal, flares, fireworks. The Particle Theory • All matter is composed of particles. These particles are called atoms. – Solids – Particles in a solid are packed tightly together and locked firmly into position. They can vibrate in position but cannot move. – Liquids – Particles in liquids are packed tightly together, but they can slide over each other and shift position. – Gases – Particles of gases have little affinity for each other and move freely about. Particles of a solid packed tightly together, cannot move. Particles of a liquid, can move over each other. Particles of a gas. Free to move about. Rapid movement. Electron Shells Electron Shells (contd.) • The lowest energy level is the “K” shell, it is the nearest to the nucleus. Electrostatic attraction at this level is greatest for the electrons. • As we move away from the nucleus into higher energy levels, nuclear attraction becomes less. (See “Atomic Size” power point file with this unit) Nobel Gases Nobel gases have complete outer electron shells. Because of this, they are Inert or Unreactive. Nobel gases are the only elements that can exist as single atoms and are written Ar, Ne, He, etc.. Gases such as nitrogen, oxygen, etc. must form molecules of two to remain stable. Groups of two atoms are called diatomic molecules. O2, Cl2, N2, etc. Are all diatomic molecules. Bonding in Compounds • Ionic Bonds – Form when atoms lose or gain electrons. Ionic bonds form due to electrostatic attraction of the ions. • Covalent Bonds – Form when atoms share electrons. – Equal Sharing – Equal sharing forms covalent molecular* compounds. – Unequal Sharing – forms slightly polar compounds call polar covalent compounds. *Molecules are electrically neutral arrangements of at least two atoms formed from strong covalent bonds. Bonding in Compounds (contd.) Lewis electron dot diagrams show only the outer shell of atoms and illustrate bonding. The lone electron of the hydrogen atom is shown as an “x”. Oxygen has six electrons shown as a “dot”. The molecule of water is shown, with the oxygen sharing electrons with hydrogen and vice versa. Oxygen is a highly electronegative atom and holds the electrons donated by hydrogen closely to itself. This produces a Polar Molecule, as shown to the left. • Lewis electron dot diagrams of ionic substances is slightly different. Na * + * Mg * + Cl O [ Na ]+ [Cl] [Mg] 2+ [O] 2- Questions 1. In terms of the particle theory, what is the difference between solids, liquids and gases. 2. In terms of size and bonding, what is the difference between argon, oxygen, and helium. 3. Draw Lewis electron dot diagrams for: a. b. c. d. Carbon dioxide Nitrogen Magnesium chloride Methane