* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Amino Acids
Survey
Document related concepts
Western blot wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Protein adsorption wikipedia , lookup
Self-assembling peptide wikipedia , lookup
Citric acid cycle wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
List of types of proteins wikipedia , lookup
Bottromycin wikipedia , lookup
Metalloprotein wikipedia , lookup
Peptide synthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Proteolysis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Genetic code wikipedia , lookup
Transcript
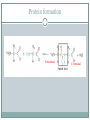
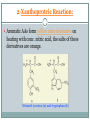
Proteins and Amino Acids BAHIYA OSRAH EMAIL: [email protected] Introduction The amino acids are the building blocks of Proteins Proteins are the fundamental structural components of the body Enzymes Contractile proteins in the muscles to permit movement Collagen in the bones Plasma albumin, hemoglobin in blood Immunoglobin to destroy infectious bacterial and viruses Storage proteins (ferrin that bind to iron and help in its storage) Maintain the osmotic pressure Introduction More than 300 different amino acids are known in nature Only 20 are commonly found as constituents of mammalian proteins Amino acids structure • At physiologic PH= 7.6 the amino acids exists as Zwitterion • The carboxyl group dissociate to form coo – • The amino group is protonate to form NH3+ Zwitterion Protein formation N terminal C terminal Physical properties Colorless Crystalline More soluble in water than in polar solvents They have high melting points more than 200 C Nutritional classification of amino acids Essential amino acids: Non-essential amino acids Cannot be synthesized Can be made by the by the body Must be taken from the diet Ex: Valine, leucine, isoleucine, phenylalanine, and tryptophan body Classification according to amino acids side chain Qualitative Tests for Amino Acids 1- Ninhydrin Reaction: A color reaction given by amino acids and peptides on heating with the chemical ninhydrin. The technique is widely used for the detection and quantitation (measurement) of amino acids and peptides. Ninhydrin is a powerful oxidizing agent which reacts with all amino acids between pH 4-8 to produce a purple colored-compound. 1- Ninhydrin Reaction The reaction is also given by primary amines and ammonia but without the liberation of Co2 The amino acids proline and hydroxyproline also reacts but produce a yellow color. Alpha-amino acid + 2 ninhydrin ---> CO2 + aldehyde + final complex (purple) + 3H2O 1- Ninhydrin Reaction Method: 1 ml AA + 1 ml NH---- heat in boiling WB for 5min----Purple color 2-Xanthoproteic Reaction: This reaction is specific for aromatic amino acids This reaction involves the nitration of benzene nucleus in alkaline medium L-Phenylalanine L-Tryptophan L-Tyrosine 2-Xanthoproteic Reaction: Aromatic AAs form yellow nitro derivative on heating with conc. nitric acid, the salts of these derivatives are orange. Nitrated tyrosine (a) and tryptophan (b) 2-Xanthoproteic Reaction: Method: 1 ml AA + 1 ml conc. HNO3----- heat the mixture in WB for 30s--cool--add drop-wise 40% NaOH to render the solution alkaline--- Yellow to orange color. 3- Millon Reaction: This reaction is used to detect the presence of phenol (hydroxybenzene) which reacts with Millon's reagent to form red complexes. - The only phenolic AA is tyrosine. L-Tyrosine 3- Millon Reaction: Method: 1 ml AA + 5 drops of Millon reagent ----- heat the mixture in BWB for 10min--cool too room temp-add 5 drops of NaNO2---Brick red color. 4- Hopkin-cole Reaction: This reaction is used to detect the presence of indol group L-Tryptophan The indol group of tryptophan reacts with glyoxalic acid in the presence of conc. H2SO4 to give purple color. 4- Hopkin-cole Reaction: Method: 1 ml AA + 1 ml Hopkin-cole reagent -----mix well-Carefully pour conc. H2SO4 down the side of the tube so as to form two layers --Purple ring at the interface. 5- Sulfur Test: This reaction is specific to detect the presence of sulfur -The sulfur of cystein and cystine is converted to inorganic sulfide with conc. NaOH. Lead acetate is added and a ppt of black lead sulfide indicates a +ve reaction. L-Cystein 5- Sulfur Test: Method: 2 ml AA + 1 ml 40% NaOH + 1-3 drops of lead acetate solution----- heat the mixture in WB for 3min -----cool--observe any change ---- Black ppt. 6-Sakaguchi Reaction: This reaction is used to detect the presence of guanidine group. L-Arginine -The only AA that contains guanidine group is arginine which reacts with α-naphthol and an oxidizing agent such as bromide water to give a red color 6-Sakaguchi Reaction: Method: 2 ml AA + 1 ml 2M NaOH + 1 ml ethanolic 0.02% αnaphthol ----- mix wellcool in ice-----add 1 ml of alkaline hypochlorite solution---- Red color