* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cough, fever and weight loss in a young male
Survey
Document related concepts
Adaptive immune system wikipedia , lookup
Rheumatic fever wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Neonatal infection wikipedia , lookup
Infection control wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Pathophysiology of multiple sclerosis wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Common cold wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Innate immune system wikipedia , lookup
Hepatitis B wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
X-linked severe combined immunodeficiency wikipedia , lookup
Transcript
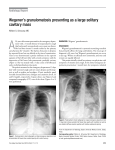
Copyright #ERS Journals Ltd 2002 European Respiratory Journal ISSN 0903-1936 Eur Respir J 2002; 19: 1210–1213 DOI: 10.1183/09031936.02.00283802 Printed in UK – all rights reserved CASE FOR DIAGNOSIS Cough, fever and weight loss in a young male R. Summer*, J. Zacks#, M. Ieong*, A. O9Regan* Case history A 48-yr-old male was admitted with 6 months of nonproductive cough, fever, and weight loss. These symptoms progressed despite a course of antibiotics prescribed for presumed community-acquired pneumonia 2 months prior to admission. A chest radiograph taken at that time is shown in figure 1a. The patient denied any other symptoms, including skin rash, a) b) Fig. 1. – a) Normal chest radiograph 2 months prior to admission. b) Admission chest radiograph. Large nodules are seen in both lungs. joint pain, and extremity numbness and weakness. Past medical history was significant for infection with Hepatitis-C virus and an accidental shotgun wound. The patient had a 30-pack-yr smoking history, remote use of injected heroin and cocaine and distant ethanol abuse. There was no occupational exposure to asbestos or silica, no recent travel and he had no pets or hobbies. He was not taking any medications. On physical examination the patient was diaphoretic with a temperature of 39.4u C, pulse 107 beat?min-1, blood pressure 133/75 mmHg, respiration 28 breaths?min-1 and oxygen saturation of 88% on room air. Chest examination revealed bilateral basilar crackles. A 2-cm lymph node that was firm, mobile and not tender was palpated in the right axilla. The rest of the examination was normal. Laboratory studies demonstrated a white blood count of 2.46109 L-1 (53% lymphocytes, 25% neutrophils, 15% monocytes, 3% eosinophils and 2% bands), haematocrit of 30% and platelet count 77610 10 L-1. Serum chemistries, renal and liver function tests were normal. Lactate dehydrogenase was normal. The patient was human immunodeficiency virus (HIV) negative. Chest radiography (fig. 1b) was performed on admission, followed by computed tomography (CT) scan of the chest (fig. 2). Over the course of the next week repeat blood, urine, and sputum cultures were sterile and a bone marrow aspirate and biopsy was nondiagnostic. A biopsy of the right axillary lymph node was performed (fig. 3). Fig. 2. – Chest computed tomography scan. *The Pulmonary Centre and and #The Dept of Pathology, Boston University School of Medicine, Boston, MA, USA. Correspondence: A. O9Regan, Assistant Professor of Medicine, The Pulmonary Center, Boston University School of Medicine, 715 Albany Street, Boston, MA 02118, USA. Fax: 1 6175368093. E-mail: [email protected] COUGH, FEVER AND WEIGHT LOSS IN A YOUNG MALE a) b) c) d) 1211 Fig. 3. – a) The dissected lymph node stained with haemotoxylin and eosin. b) The same section under higher magnification. Atypical lymphocytes are highlighted by solid arrows. c) B-lymphocytes are stained with CD20 marker (brown cells are indicated by an open arrow). Abundant T-lymphocyte population (solid arrow) surrounds the CD20 positive cells. d) Positive staining for small Ebstein-Barr encoded ribonucleic acid particles. Scale bars=50 mm. BEFORE TURNING THE PAGE, INTERPRET THE CHEST RADIOGRAPHS (FIGS. 1a AND b), THE COMPUTED TOMOGRAPHY SCAN (FIG. 2) AND THE PHOTOMICROGRAPH (FIG. 3), AND SUGGEST A DIAGNOSIS. 1212 R. SUMMER ET AL. Interpretations Chest radiographs The frontal chest radiograph (fig. 1a) taken 2 months prior to admission is normal. The small circular radio-opaque nodules in the chest represent metallic pellets from a prior shotgun accident. The repeat film on admission (fig. 1b) demonstrates large nodules of variable size predominantly involving the lower lung fields. Computed tomography The chest CT scan (fig. 2) shows multiple nodular masses throughout the lungs. Nodules were not calcified and there was no cavitation. There was no mediastinal adenopathy or pleural disease. Pathology Figure 3a shows the dissected lymph node (magnification610), demonstrating a nodular perivascular infiltrate with extensive tissue necrosis consistent with angiitis and granulomatosis. No epithelioid cells or granulomas are seen. In figure 3b, higher magnification of the perivascular infiltrate confirms the presence of polymorphic lymphocytic cells with occasional large atypical mononuclear cells. Figure 3c demonstrates, by immunohistochemistry, that the large atypical cells express B-cell (CD20) antigens. Deoxyribonucleic acid extracted from the tissue and amplified using the polymerase chain reaction demonstrated a rearrangement of the immunoglobulin heavy chain, consistent with a monoclonal B-cell population (not shown). As shown in figure 3d, the large cells also demonstrate nuclear staining for small Ebstein-Barr encoded ribonucleic acid particles (EBERs) by in situ hybridization. Diagnosis: Lymphomatoid granulomatosis Clinical course The patient was treated with a chemotherapy regimen of cyclophosphamide, doxorubicio, Oncovin and prednisone (CHOP). Despite aggressive treatment, progressive respiratory failure ensued and the patient died˜ 1 week after the initiation of chemotherapy. Discussion Lymphomatoid granulomatosis (LYG) is a multisystem angiocentric lymphoproliferative disease with a predilection for the lung, skin and nervous system. LYG was originally classified among a benign group of diseases characterized by pulmonary angiitis (perivascular inflammation) and granulomatosis (central necrosis rather than granuloma formation), including Wegener9s granulomatosis, Churg-Straus syndrome, and bronchocentric granulomatosis [1]. Recent advances have demonstrated that LYG is, in fact, a B-cell lymphoma related to Epstein-Barr virus (EBV) infection [2, 3]. New approaches to diagnosis and therapy are being studied. Diagnosis requires the presence of a triad of histological findings on biopsy specimens usually obtained from the lung: a polymorphic lymphocytic infiltrate, angiitis, and granulomatosis [1]. LYG is a destructive lesion due to vessel occlusion by lymphoid infiltration and subsequent necrosis. New molecular testing has shown that LYG lesions consist of clonally-expanded B-cells surrounded by a prominent polyclonal reactive T-cell infiltrate [3]. Thus, LYG is now considered to be a T-cell rich B-cell lymphoma. A strong association exists between LYG and infection with EBV [3, 4]. As in transplant and HIVassociated lymphoma, in most cases of LYG, EBV RNA is present in the clonally-expanded B-cells. The role of opportunistic pathogens in the development of LYG is further supported by the increased occurrence of LYG in various forms of immune dysregulation. LYG has been reported in patients with autoimmune diseases, HIV infection, transplantationassociated immunosuppression and primary immune deficiencies such as Wiskott-Aldrich syndrome [5–7]. Furthermore, even LYG patients who are believed to have normal immune systems have been found to have subtle immunological abnormalities, such as a decreased number of CD8 cells or defective delayedtype hypersensitivity responses. Thus, the pathogenesis of LYG appears to involve opportunistic infection of a susceptible host. LYG typically presents in the fifth and sixth decades of life, with a slight male predominance. Fever, cough, malaise and weight loss are common symptoms and the most frequently affected organs are the lung (90%), skin (40%), and nervous system (25%) [4, 8]. Spleen and lymph nodes are rarely involved. No laboratory finding are consistently found. Chestradiography findings include bilateral nodular lesions (80–100%), which may rarely cavitate and predominantly involve the lower and peripheral lung fields. Pleural effusions are present in one-third of patients. The prognosis is quite poor with a median survival of 14 months. The cause of death is usually from respiratory failure or sepsis. Transformation into high-grade lymphoma occurs in 13–49%. Less than 10% of cases resolve spontaneously [4, 8]. Previously, lymphomatoid granulomatosis was treated with immunosuppressive medications, such as glucocorticoids and cyclophosphomide. Recent case series have used therapeutic approaches aimed at improving rather than suppressing immune function. Moreover, suppressing viral replication may be an effective alternative. For instance, two trials of interferon-2a for the treatment of lymphomatoid granulomatosis have demonstrated dramatic clinical responses [9, 10]. Further studies using drugs that augment the immune system or suppress viral replication are required. COUGH, FEVER AND WEIGHT LOSS IN A YOUNG MALE References 1. 2. 3. 4. 5. Liebow AA, Carington CRB, Friedman PJ. Lymphomatoid granulomatosis. Hum Pathol 1972; 3: 457– 558. Tanière Ph, Thivolet-Béjui F, Vitrey D, et al. Lymphomatoid granulomatosis - a report on four cases: evidence for B phenotype of the tumor cells. Eur Respir J 1998; 12: 102–106. Katzenstein AL, Peiper SC. Detection of Epstein-Barr virus genomes in lymphomatoid granulomatosis: analysis of 29 cases by the polymerase chain reaction technique. Mod Pathol 1990; 3: 435–441. Fauci AS, Haynes BF, Costa J, Katz P, Wolff SM. Lymphomatoid granulomatosis. Prospective clinical and therapeutic experience over 10 years. NEJM 1982; 306: 68–74. Haque AK, Myers JL, Hudnall SD, et al. Pulmonary lymphomatoid granulomatosis in acquired immunodeficiency syndrome: lesions with Epstein-Barr virus infection. Mod Pathol 1998; 11: 347–356. 6. 7. 8. 9. 10. 1213 Howite NT, Fligner CL, Ochs HD, et al. Pulmonary angiitis with atypical lymphoreticular infiltrates in Wiskott-Aldrich syndrome: possible relationship of lymphomatoid granulomatosis and EBV infection. Clin Immunol Immunopathol 1986; 41: 479–484. Fassas A, Jagannath S, Desikan KR, et al. Lymphomatoid granulomatosis following autologous stem cell transplantation. Bone Marrow Transplant 1999; 23: 79–81. Katzenstein LA, Carrington CB, Liebow AA. Lymphomatoid granulomatosis. A clinicopathologic study of 152 cases. Cancer 1979; 43: 360–373. Wilson WH, Kingma DW, Raffeld M, Wittes RE, Jaffe ES. Association of lymphomatoid granulomatosis with Ebstein-Barr viral infection of B lymphocytes and response to interferon-alpha-2b. Blood 1996; 87: 4531–4537. Richter C, Schnabel A, Muller KM, Reuter M, Schuster P, Gross WL. Lymphomatoid granulomatosis - remission with interferon-alpha-2b. Dtsch Med Wochenschr 1997; 122: 1106–1110.