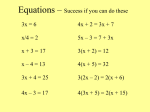* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemical Equations - Salem Community Schools
Atomic theory wikipedia , lookup
Organic chemistry wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Drug discovery wikipedia , lookup
Fine chemical wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Asymmetric induction wikipedia , lookup
Marcus theory wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
History of chemistry wikipedia , lookup
Safety data sheet wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Process chemistry wikipedia , lookup
Rate equation wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Chemical potential wikipedia , lookup
Chemical weapon wikipedia , lookup
Chemical Corps wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Chemical plant wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Chemical industry wikipedia , lookup
Electrochemistry wikipedia , lookup
Click chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Chemical reaction wikipedia , lookup
Transition state theory wikipedia , lookup
VX (nerve agent) wikipedia , lookup
Chemical Reactions and Equations: Basic Concepts Recognizing Chemical Reactions • When a substance undergoes a chemical change, it takes part in a chemical reaction. • After it reacts, it no longer has the same chemical identity. Chemical Reactions and Equations: Basic Concepts Recognizing Chemical Reactions • While it may seem amazing that a substance can undergo a change and become part of a different substance, chemical reactions occur around you all the time. • Many important clues indicate when chemical reactions occur. • None of them alone proves that such a change occurs because some physical changes involve one or more of these signs. Chemical Reactions and Equations: Basic Concepts Writing Chemical Equations • In order to completely understand a chemical reaction, you must be able to describe any changes that take place. • Part of that description involves recognizing what substances react and what substances form. Chemical Reactions and Equations: Basic Concepts Writing Chemical Equations • A substance that undergoes a reaction is called a reactant. • When reactants undergo a chemical change, each new substance formed is called a product. Chemical Reactions and Equations: Basic Concepts Writing Chemical Equations • For example, a familiar chemical reaction involves the reaction between iron and oxygen (the reactants) that produces rust, which is iron(III) oxide (the product). • The simplest reactions involve a single reactant or a single product, but some reactions involve many reactants and many products. Chemical Reactions and Equations: Basic Concepts Word Equations • The simplest way to represent a reaction is by using words to describe all the reactants and products, with an arrow placed between them to represent change. • Reactants are placed to the left of the arrow, and products are placed to the right. • Plus signs are used to separate reactants and also to separate products. Chemical Reactions and Equations: Basic Concepts Word Equations • Vinegar and baking soda are common names. • The compound in vinegar that is involved in the reaction is acetic acid, and baking soda is sodium hydrogen carbonate. • These scientific names can also be used in a word equation. Chemical Reactions and Equations: Basic Concepts Chemical Equations • Word equations describe reactants and products, but they are long and awkward and do not adequately identify the substances involved. • Word equations can be converted into chemical equations by substituting chemical formulas for the names of compounds and elements. Chemical Reactions and Equations: Basic Concepts Chemical Equations • The equation for the reaction of vinegar and baking soda can be written using the chemical formulas of the reactants and products. • By examining a chemical equation, you can determine exactly what elements make up the substances that react and form. Chemical Reactions and Equations: Basic Concepts • • • • Chemical Equations It may also be important to know the physical state of each reactant and product. How can we indicate the bubbles we see during this reaction are CO2? Symbols in the parentheses are put after formulas to indicate the state of the substance. Solids, liquids, gases, and water (aqueous) solutions are indicated by the symbols (s), (l), (g), and (aq). Chemical Reactions and Equations: Basic Concepts Chemical Equations • The following equation shows these symbols added to the equation for the reaction of vinegar and baking soda. Chemical Reactions and Equations: Basic Concepts Chemical Equations • Now the equation tells us that mixing an aqueous solution of acetic acid (vinegar) with solid sodium hydrogen carbonate (baking soda) results in the formation of an aqueous solution of sodium acetate, liquid water, and carbon dioxide gas. Chemical Reactions and Equations: Basic Concepts Energy and Chemical Equations • Noticeable amounts of energy are often released or absorbed during a chemical reaction. • Some reactions absorb energy. If energy is absorbed, the reaction is known as an endothermic reaction. • For a reaction that absorbs energy, the word energy is sometimes written along with the reactants in the chemical equation. Chemical Reactions and Equations: Basic Concepts Energy and Chemical Equations • For example, the equation for the reaction in which water breaks down into hydrogen and oxygen shows that energy must be added to the reaction. Chemical Reactions and Equations: Basic Concepts Energy and Chemical Equations • Reactions that release heat energy are called exothermic reactions. Chemical Reactions and Equations: Basic Concepts Energy and Chemical Equations • When writing a chemical equation for a reaction that produces energy, the word energy is sometimes written along with the products. • Some of this energy is in the form of light. Chemical Reactions and Equations: Basic Concepts Energy and Chemical Equations • You may have also noticed that the word energy is not always written in the equation. • It is used only if it is important to know whether energy is released or absorbed. Chemical Reactions and Equations: Basic Concepts Balancing Chemical Equations • The mass of the products is always the same as the mass of the reactants that react to form them. • The law of conservation of mass summarizes these findings. • Matter is neither created nor destroyed during a chemical reaction. Chemical Reactions and Equations: Basic Concepts Balancing Chemical Equations • Remember that atoms don’t change in a chemical reaction; they just rearrange. • The number and kinds of atoms present in the reactants of a chemical reaction are the same as those present in the products. • When stated this way, it becomes the law of conservation of atoms. Chemical Reactions and Equations: Basic Concepts Balancing Chemical Equations • For a chemical equation to accurately represent a reaction, the same number of each kind of atom must be on the left side of the arrow as are on the right side. • If an equation follows the law of conservation of atoms, it is said to be balanced. Chemical Reactions and Equations: Basic Concepts Balancing Chemical Equations • The easiest way to count atoms is to practice—first with a simple reaction and then with some that are more complex. • For example, consider the equation that represents breaking down carbonic acid into water and carbon dioxide. Chemical Reactions and Equations: Basic Concepts Balancing Chemical Equations • Because a subscript after the symbol for an element represents how many atoms of that element are found in a compound, you can see that there are two hydrogen, one carbon, and three oxygen. • All of the atoms in the reactants are the same as those found in the products. Chemical Reactions and Equations: Basic Concepts Balancing Chemical Equations • Examine the equation for the formation of sodium carbonate and water from the reaction between sodium hydroxide and carbon dioxide. Chemical Reactions and Equations: Basic Concepts Balancing Chemical Equations • One carbon atom is on each side of the arrow, but the sodium, oxygen, and hydrogen atoms are not balanced. • The equation, as written, does not truly represent the reaction because it does not show conservation of atoms. Chemical Reactions and Equations: Basic Concepts Balancing an Equation • To indicate more than one unit taking part or being formed in a reaction, a number called a coefficient is placed in front of it to indicate how many units are involved. • Look at the previous equation with a coefficient of 2 in front of the sodium hydroxide formula. Chemical Reactions and Equations: Basic Concepts • • • • • Balancing an Equation Is the equation balanced now? Two sodium atoms are on each side. How many oxygen atoms are on each side? You should be able to find four on each side. How about hydrogen atoms? Now two are on each side. Because one carbon atom is still on each side, the entire equation is balanced; it now represents what happens when sodium hydroxide and carbon dioxide react. Chemical Reactions and Equations: Basic Concepts Balancing an Equation • The balanced equation tells us that when sodium hydroxide and carbon dioxide react, two units of sodium hydroxide react with each molecule of carbon dioxide to form one unit of sodium carbonate and one molecule of water. Chemical Reactions and Equations: Basic Concepts Major Classes of Reactions • If you can classify a reaction into one of five major categories by recognizing patterns that occur, you already know a lot about the reaction. Chemical Reactions and Equations: Basic Concepts Major Classes of Reactions • In one type of reaction, two substances— either elements or compounds—combine to form a compound. • Whenever two or more substances combine to form a single product, the reaction is called a synthesis reaction. Chemical Reactions and Equations: Basic Concepts A Synthesis Reaction • When iron rusts, iron metal and oxygen gas combine to form one new substance, iron(III) oxide. • The balanced equation for this synthesis reaction shows that there is more than one reactant but only one product. Chemical Reactions and Equations: Basic Concepts A Synthesis Reaction Chemical Reactions and Equations: Basic Concepts Major Classes of Reactions • In a decomposition reaction, a compound breaks down into two or more simpler substances. • The compound may break down into individual elements, such as when mercury(II) oxide decomposes into mercury and oxygen. Chemical Reactions and Equations: Basic Concepts Major Classes of Reactions • The products may be an element and a compound, such as when hydrogen peroxide decomposes into water and oxygen. • The compound may break down into simpler compounds. Chemical Reactions and Equations: Basic Concepts A Decomposition Reaction • When ammonium nitrate is heated to a high temperature, it explosively breaks down into dinitrogen monoxide and water. • The decomposition reaction taking place is represented by a balanced equation that shows one reactant and more than one product. Chemical Reactions and Equations: Basic Concepts A Decomposition Reaction Chemical Reactions and Equations: Basic Concepts Major Classes of Reactions • In a single-displacement reaction, one element takes the place of another in a compound. • The element can replace the first part of a compound, or it can replace the last part of a compound. Chemical Reactions and Equations: Basic Concepts Single Displacement • If an iron nail is placed into an aqueous solution of copper(II) sulfate, the iron displaces the copper ions in solution, and copper metal forms on the nail. Chemical Reactions and Equations: Basic Concepts Single Displacement Chemical Reactions and Equations: Basic Concepts Major Classes of Reactions • In double-displacement reactions, the positive portions of two ionic compounds are interchanged. • For a double-displacement reaction to take place, at least one of the products must be a precipitate or water. Chemical Reactions and Equations: Basic Concepts Double Displacement • When clear aqueous solutions of lead(II) nitrate and potassium iodine are mixed, a double-displacement reaction takes place and a yellow solid appears in the mixture. • This solid is lead(II) iodine, and it precipitates out because it is insoluble in water, unlike the two reactants and the other product. Chemical Reactions and Equations: Basic Concepts Double Displacement Chemical Reactions and Equations: Basic Concepts Major Classes of Reactions • A combustion reaction is one in which a substance rapidly combines with oxygen to form one or more oxides. Chemical Reactions and Equations: Basic Concepts Combustion • When welding is done with an acetylene torch, acetylene combines with oxygen to form carbon dioxide and water. • This combustion reaction is exothermic, and enough energy is released to melt metal. Chemical Reactions and Equations: Basic Concepts Combustion Chemical Reactions and Equations: Additional Concepts Reactions in Aqueous Solutions • When aqueous solutions that contain ions are mixed, the ions may react in a doublereplacement reaction. • The product is typically a solid precipitate, water, or a gas. Chemical Reactions and Equations: Additional Concepts Reactions in Aqueous Solutions • An example of a double-replacement reaction that produces a precipitate occurs when aqueous solutions of sodium chloride and silver nitrate are mixed to form a precipitate of solid silver chloride. Chemical Reactions and Equations: Additional Concepts Reactions in Aqueous Solutions • To show all of the particles in solution as they really exist, a complete ionic equation can be written. • The sodium and nitrate ions are on both sides of the equation. • Such ions that do not participate in a reaction are called spectator ions. Chemical Reactions and Equations: Additional Concepts Reactions in Aqueous Solutions • An ionic equation that does not show spectator ions but only the particles that participate in a reaction is called a net ionic equation. • In the case of the reaction above, the net ionic equation from which the sodium and nitrate ions have been removed is as follows. Chemical Reactions and Equations: Additional Concepts Writing Ionic Equations • Write the balanced chemical equation for the reaction between aqueous solutions of strontium nitrate and potassium sulfate, which forms the precipitate strontium sulfate. • Then write the complete ionic and net ionic equations. Chemical Reactions and Equations: Additional Concepts Writing Ionic Equations • Write the correct skeleton equation. • Use coefficients to produce the balanced chemical equation. Chemical Reactions and Equations: Additional Concepts Writing Ionic Equations • Write the complete ionic equation. Chemical Reactions and Equations: Additional Concepts Writing Ionic Equations • Cross out the spectator ions, which are those that are on both sides of the equation. • That leaves the net ionic equation. Chemical Reactions and Equations: Additional Concepts • • • • Reactions that form water or a gas Some double-replacement reactions in aqueous solution produce water or a gas (or both) rather than a precipitate. In such cases, the water or gas is shown as a product in the net ionic equation, as are the ions that produced it. The remaining ions are eliminated as spectator ions. The following example problem illustrates this concept. Chemical Reactions and Equations: Additional Concepts Reactions that form water or a gas • When hydrochloric acid and potassium hydroxide solutions are mixed, water results, together with an aqueous solution of potassium chloride. • Write the balanced chemical equation, a complete ionic equation, and a net ionic equation for this reaction. Chemical Reactions and Equations: Additional Concepts Reactions that form water or a gas • The balanced chemical equation is the same as the skeleton equation. Chemical Reactions and Equations: Additional Concepts Reactions that form water or a gas • Write the complete ionic equation, which includes all of the ions. • Remove the spectator ions to produce the net ionic equation.