* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Spin Angular Momentum Magnetic Moments
Molecular Hamiltonian wikipedia , lookup
Scalar field theory wikipedia , lookup
Canonical quantization wikipedia , lookup
Ising model wikipedia , lookup
Nitrogen-vacancy center wikipedia , lookup
Hydrogen atom wikipedia , lookup
Magnetic monopole wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Magnetoreception wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Mössbauer spectroscopy wikipedia , lookup
Electron paramagnetic resonance wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Spin (physics) wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
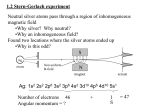
Nuclear Magnetism and NMR Spectroscopy - Ralph W. Adams Lecture 1: Nuclear Magnetic Resonance – Theory and Techniques Spin Angular Momentum Magnetic nuclei possess an intrinsic angular momentum known as spin. Spin is quantized and has units ħ (where ħ = h / 2π and h is the Planck constant, 6.63 x 10-34 m2 kg s-1). I Nuclide 12 The magnitude of spin angular momentum is �I (I + 1) ħ. 0 C, 16O 1 1/2 H, 13C, 15N, 29Si, 31P The spin quantum number I can take a value I = 0, 1/2 , 1, 3/2, 2, 5/2, … 2 1 H, 14N 11 When discussing NMR we often use a special subset of language: 3/2 B, 23Na, 35Cl, 37Cl 17 2 O, 27Al spin means magnetic nucleus 10 5/2 B protons is often used in place of 1H nuclei The spin quantum number, and proof of the quantum mechanical nature of spin, can be measured and shown by performing a Stern-Gerlach experiment. Here, a beam of spins is passed through a magnetic field and onto a detector. The number of positions of signal detected indicated the spin quantum number while the lack of a continuum between points at which spins land shows that the system is quantized. Spin angular momentum can be described using a vector I whose direction and magnitude are quantized. The length of I is �I (I +1) ħ with 2I + 1 projections along the axis of the applied magnetic field. By convention the axis along which the magnetic field is applied is labelled z. The projection of I onto the z axis is labelled Iz. Iz = m ħ where m = I, I - 1, I - 2, …, - I. Magnetic Moments The magnetic moment µ is related to the spin angular momentum I by µ = γ I where γ is the magnetogyric ratio of the nuclide. Nuclide γ ( 106 rad s−1 T−1) ν (MHz T−1) H 1 267.513 42.58 2 H 41.065 6.54 13 C 67.262 10.71 15 −27.116 −4.32 19 251.662 40.05 29 Si −53.190 −8.47 P 108.291 17.24 N F 31 Figure 1. Space quantization of the angular momentum of spin-½ and spin-1. Magnetic moment is parallel to spin angular momentum (if γ is positive, antiparallel if γ is negative). The magnitude and orientation of γ and I are quantized. Nuclear Magnetism and NMR Spectroscopy - Ralph W. Adams Lecture 1: Nuclear Magnetic Resonance – Theory and Techniques Energy levels If we introduce a magnetic field B – a vector because it has direction and magnitude – then we can calculate classically the energy E of a magnetic moment µ by taking the scalar product of the vectors µ and B. E=-µ∙B If the magnetic field is strong then the spin quantization axis coincides with the field direction. E = - µz B where µz is the z component of µ. As µz = γ Iz = γ m ħ, E = - γ m ħ B. This means that the energy of the nucleus is changed in proportion with the magnetic field strength B, the magnetogyric ratio γ, and the z-component of the spin angular momentum. The energy gap ΔE = γ ħ B with a corresponding frequency νNMR = (γ B)/(2 π). Resonance frequencies and chemical shift The magnetic field within an atom differs slightly from the external field, depending on the chemical environment. This is the origin of chemical shift (δ). At 9.4 T (T = Tesla, the SI unit of magnetic field strength), c. 100,000 stronger than the earth’s field, 2.675 x 108 rad s-1 T-1 x 9.4 T 2π 6 = 400.2 x 10 Hz Larmor frequency, νNMR = (γ B)/(2 π) = νNMR is in the radiofrequency part of the electromagnetic spectrum so we use the term R.F. field when discussing the radiation required to irradiate NMR transitions. We usually refer to the 1H NMR frequency rather than the magnetic field as 1H is the most commonly studied nucleus. For uncoupled spins, the description of the NMR experiment can be easily described by rotating magnetisations. Individual spins are not observed in a normal NMR experiment, rather it is a bulk magnetization vector – the sum of all of the spin angular moments – which is manipulated and observed. The bulk magnetization vector cannot be observed when it is directed along the z axis. For a basic NMR experiment a pulse of electromagnetic radiation at an appropriate radiofrequency is applied to rotate the bulk magnetisation vector into the transverse plane – conventionally labelled x,y. This allows Larmor precession – rotation around the z axis at the frequency νNMR – to be observed, and it is this precession which is used to generate an NMR signal. The behaviour of an uncoupled spin Nuclear Magnetism and NMR Spectroscopy - Ralph W. Adams Lecture 1: Nuclear Magnetic Resonance – Theory and Techniques can be approximated to the behaviour of a classical rotor such as a gyroscope or bicycle wheel and the mathematics associated with these types of systems are applicable to many NMR systems. The expanded region of the NMR spectrum (at 9.4 T) shows how chemical shifts and NMR spectroscopy fit into the full electromagnetic spectrum. Populations and polarizations We can calculate a Boltzmann distribution across the lower (m = + ½) and upper (m = - ½) energy levels for 1H spins. There is a convention of naming the lower and upper energy levels α and β respectively. For nuclei with spin I = 1/2, the ratio of the number of nuclei in the upper energy level β to the number of nuclei in the lower energy level α is determined by Boltzmann’s equation: nβ / nα = exp(-ΔE / kBT) ΔE = γ ħ B = 2.65 x 10-25 J and kBT = 4.14 x 10-21 J, so ΔE/kBT = 6.4 x 10-5 This means that the available thermal energy, kBT, is large compared to the energy required to reorient the spins and that the population difference will be small. For this example it can be shown that the ratio nβ / nα corresponds to nα = 15625 and nβ = 15624, so the population difference is only 1 in 31250 molecules. The result here shows that the equivalent of only 1 in 104 – 106 nuclei (depending on γ, B, and T) will contribute to the observed NMR signal – NMR is a very weak phenomenon.