* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Introduction
Genome (book) wikipedia , lookup
Gene expression profiling wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Quantitative trait locus wikipedia , lookup
Microevolution wikipedia , lookup
Oncogenomics wikipedia , lookup
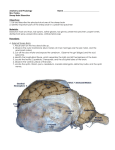
Effects of Immunization Against Bone Morphogenetic Protein 15 (BMP-15) and Growth Differentiation Factor 9 (GDF-9) on Some Selected Reproductive Trait Presented by EMAD M. A. SAMARA Introduction • Multiple ovulation – Complex trait influenced by genetic and environmental factors – Ovulation rate • Number of mature oocytes released during one reproductive cycle • Low vs. High ovulation rate phenotype – Primates and ruminants vs. mice and pigs • Number of follicles that mature within species is tightly regulated – Ensure optimal fertility and maximum survival of offspring Introduction • Current models of follicular selection indicate that multiple ovulation is controlled by – Ovarian-pituitary gland • Determined by a complex exchange of endocrine signals between the pituitary gland and the ovary – Concentrations of FSH/LH near the time of follicular selection/dominance – Intra-ovarian factors • Para-crine and auto-crine signals within ovarian follicles involving the oocyte and its adjacent cumulus cells (CCs) cells Introduction • Oocyte Developmental Competence (ODC) – Capacity of a mature oocyte to support the very earliest stages of life, fertilization, pre-implantation embryo development and implantation, – Measure the intrinsic oocyte quality – Affect by many factors 1. Oocyte origin 1. Oocytes derived from large follicles are more competent than those derived from small follicles 2. Follicle health 1. Follicle dominance and atresia are associated with developmental competence 3. Hormonal stimulation of follicle development 4. Communication between the oocyte and its surrounding CCs 1. Oocyte is dependent on CCs to provide nutrients and regulatory signals which are necessary to promote oocyte nuclear and cytoplasmic maturation and hence the acquisition of developmental competence Introduction • One approach to understanding critical pathways in this complex regulatory systems is to find natural and induced mutations that influence the target phenotype. • Sheep have proved to be a valuable model for the study of follicular growth and selection. – Highly diverse species (more than 900 breeds) that vary greatly in their physiological characteristics including ovulation rate and fecundity which influenced by genetic background and the effects of age, season and nutrition. – A number of natural genetic mutations have been identified with ovulation rates varying between 0 and 15. Introduction • Genome scanning projects have been initiated for identification of genes controlling many quantitative traits in sheep. – Preliminary reports of the identification of sheep quantitative trait loci (QTLs) include parasite resistance, wool production traits, milk traits, carcass traits, out-of-season breeding, and dagginess – Obstacles • Only preliminary results • Production of suitable resource families for QTL searches has required a minimum of 3–5 years. • Identification of genes associated with ovine QTL are dependent on the study of candidate genes revealed in other species. Introduction • Solution – Identification of Single Gene Traits in Sheep • Chromosomal assignment of many single-gene traits in sheep are known, including the Booroola and Inverdale fecundity gene, Callipyge muscle hypertrophy, and Double muscling. • Current knowledge of Major genes for prolificacy in sheep falls into three categories – Genes where the mutation has been identified and DNA testing is available – Genes where the mode of inheritance has been described but the mutation has not been identified – Putative genes where there is evidence of apparent genetic segregation but there are insufficient records to ascertain the mode of inheritance Introduction • From examination of inherited patterns of ovulation rate in sheep, several breeds have been identified with mutations in two growth factor genes that are expressed in oocytes – Oocyte Secreted Factors (OSFs) • Growth Differentiation Factor-9 (GDF-9) • Bone Morphogenetic Protein-15 (BMP-15), AKA (GDF-9B) – Currently, five different point mutations have been identified in the BMP-15 gene and one in the GDF-9 gene, each having a major effect on ovulation rate • All heterozygous animals for any one of these mutations have increased ovulation rates, whereas homozygous individuals are sterile with ovarian follicular development arrested in type 2 stage of growth. Introduction • Reasons of higher ovulation rate in heterozygous for mutations in either BMP-15 or GDF-9 – Absence or extremely low concentrations of either of these factors leads to an inhibition of ovarian follicular development and thus sterility • Homozygous – whereas a partial reduction in concentrations of these factors increases ovulation rate and thus litter size – Increased FSH responsiveness and an earlier acquisition of LH receptors by the granulosa cells • BMP-15 cause suppression of FSH receptor expression and GDF-9 inhibits FSH actions in rat granulosa cells, thereby suggesting that reduced BMP-15 concentrations are associated with a higher level of FSH responsiveness Properties of BMP-15 and GDF-9 • Sheep BMP-15 – Maps to the X chromosome, – Full-length coding sequence; 1179 nucleotides encode • Pre-pro-protein region of 268 amino-acid residues • Mature protein region of 125 amino-acid residues • Sheep GDF9 – Maps to the 5 chromosome, – Full-length coding sequence; 1359 nucleotides encode • Pre-pro-protein region of 318 amino-acid residues • Mature protein region of 135 amino-acid residues • Intracellular processing results in the separation of the biologically active mature region from the pro-region – Both the mature and unprocessed pro-mature forms of BMP-15 and GDF-9 can be identified in follicular fluid and it remains to be determined what are the final forms of these proteins that regulate target cell functions. • Homodimer vs. Heterodimer Properties of BMP-15 and GDF-9 • Members of the Transforming Growth Factor-β (TGF-β) superfamily – Comprised of over 30 proteins, including TGF-β, Activin/Inhibin, Growth Differentiation Factors, Bone Morphogenetic Proteins, and Anti-Mullerian Hormone. – Controlling cellular growth, differentiation during fetal and adult life, and important for regulating ovarian follicular development. • BMP-15 and GDF-9 – Distinguishing by changing concentrations leads to incremental changes in ovulation rate in sheep. Properties of BMP-15 and GDF-9 • Within the ovary, both BMP-15 and GDF-9 mRNA and protein are localized exclusively to the oocyte. – Only exception to this is that GDF9 mRNA and protein have been localized to granulosa cells immediately adjacent to the oocyte in some primates. • In sheep, – BMP-15 mRNA and protein are not present until follicles have just begun to grow (the primary stage of development, type 2 follicle), and localized to oocytes of most, if not all, developing follicles – GDF-9 mRNA and protein can be identified in sheep oocytes during follicular formation, in primordial (type 1 follicles), and in oocytes at all stages of follicular growth Properties of BMP-15 and GDF-9 • As a members of (TGF-β) superfamily – The interaction of BMP-15 or GDF-9 with the target cell is thought to be via two types of membrane-bound serine/ threonine kinases, which has two divergent intracellular signaling pathways: • BMP-15 – Bind to a BMP type I receptor (ALK-6) and to BMPR-II – Utilizes SMAD 1/5/8 pathways • GDF-9 – Bind to a TGF-β type 1 receptor (ALK-5) and to BMPR-II – Utilizes SMAD 2/3 molecules Introduction • Since the oocyte is a major source of both BMP15 and GDF9 and the major target for these factors is the granulosa cells – It is reasonable that any interference that these OSFs modulate the responsiveness of granulosa cells to the gonadotrophins. – We therefore will asked … • Whether the intra-follicular concentrations of BMP15 or GDF9 could be modulated by exogenous means to alter ovulation rate? • Whether it is possible to mimic reliably the lambing and/or ovulation rate increases that are found in ewes that have heterozygous mutations in either BMP-15 or GDF-9? • Is it possible that if we modify the concentrations of GDF-9 and BMP-15 in sheep using an immunisation approach alter ovulation rate? The Objects • Determine the effect of long and short-term immunization against BMP-15 and GDF-9 peptides on – – – – – Various ovarian parameters Estrus behavior Ovulation Rate Fertilization of released oocytes Ability of fertilized oocytes to undergo normal fetal development – Ability of immunized ewes to carry a pregnancy to term Material and Method • Animals: – ~ 50 kg, 5- 6 yr old parous Romney ewes • Research Design : – Generation of Antigens for Sheep Immunization • Peptides : – KKPLVPASVNLSEYFC (GDF-9) ~15/16 mer (GDF-9 peptide) – SEVPGPSREHDGPESC (BMP-15) ~15/16 mer (BMP-15 peptide) – Synthesized and conjugated to keyhole limpet hemocyanin (KLH) through the C- terminal cysteine residue – Animal preparation • Ewe Synchronization – CIDR • Active immunization against GDF-9 and BMP-15 – Long-term immunization – Short-term immunization • Introducing mature Poll Dorset rams to the immunized ewes Material and Method • Research Design : – Ovarian Structural Studies – Determination of antibody titers and crossreactivity – Determination of progesterone concentrations – Statistical analysis Long-term immunization • Peptide 1 – Grouping ewes • Group 1: KLH (n = 10) • Group 2: KLH-GDF9 (15 mer GDF-9 peptide) (n = 10) • Group 3: KLH-BMP15 (15 mer BMP15 peptide) (n = 10) – The GDF-9 and BMP-15 peptides used in this study were targeted at regions of the respective proteins that are highly flexible and important for dimerisation • Peptide 2 – Grouping ewes • Group 1: KLH (n = 10) • Group 2: KLH-GDF9 (16 mer GDF-9 peptide) (n = 10) • Group 3: KLH-BMP15 (16 mer BMP15 peptide) (n = 10) – The GDF-9 and BMP-15 peptides used in this study were targeted at regions of the peptide sequence near a putative type 1 receptor binding region. Long-term immunization • The immunisation program began when the ewes were in anoestrus and continued through the breeding season. • Ewes were administered the antigen, initially in Freund’s complete adjuvant, and thereafter at monthly intervals, in Freund’s incomplete adjuvant for 7 months. – Long-term immunization (8 months) Short-term immunization • Grouping ewes – Group 1: KLH (n = 50) – Group 2: KLH-GDF9 (15 mer GDF-9 peptide) (n = 30) – Group 3: KLH-BMP15(15 mer BMP15 peptide) (n = 30) • The GDF-9 and BMP-15 peptides used in this study were targeted at regions of the respective proteins that are highly flexible and important for dimerisation • Immunisation program – Using a primary vaccination followed a month later with a single booster vaccination in a DEAE Dextran (5%, w/v) adjuvant • Short-term immunization (one month) Results • Ovarian Structure – Ovarian Morphology • Long-term immunization (peptide 1) – Neutralization of either GDF-9 or BMP-15 resulted in abnormal ovarian morphology » Presence of enlarged oocyte surrounded by a single layer of flattened and/or cuboidal granulosa cells » Normal follicular development had been impaired from the type 2 stage of growth in both the GDF9 and BMP15 treatment groups. • Short-term and Long-term immunization (peptide 2) – Like KLH alone, normal ovarian structures. – Pre-antral and Antral Follicle • Long-term immunization (peptide 1) – Arrested normal follicular development at the type 1a (transitory) or type 2 (primary) stage – Abnormal oocyte » Large for the number of layers of granulosa cell » Remnants of zona pellucida-like material » Disorganization of the granulosa or cumulus cells. • Short-term and Long-term immunization (peptide 2) – Like KLH alone, normal follicular structures. • Ovaries in ewes treated with – (A) KLH alone – (B) KLH-GDF9 peptide 1 – (C) KLH-BMP15 peptide 1 • A) Numerous or large antral follicles • B, C) inactivated ovaries • Normal and abnormal ovarian antral follicles in ewes treated with – (A, C) KLH alone – (B, D) KLH-BMP15 peptide 1 • A, C) Normal type 5 follicle • B) abnormal type 5 follicle with oocyte not completely surrounded by cumulus cells and irregular spaces within the granulosa cell layers • D) Abnormal type 5+ follicle with large holes in the cumulus cell arrangement around the oocyte and disorganized granulosa cell layer adjacent to the basement membrane. Results • Antibody Titers and Cross-Reactivity – Ewes immunized with KLH did not have measurable antibodies to either GDF-9 or BMP-15. – Ewes immunized against GDF9 had measurable antibodies against GDF9 and those immunized against BMP15 had measurable antibodies against BMP15 (P < 0.001). – Maximum cross-reactivity • Antisera collected from BMP15 immunized ewes to GDF9 – Averaged 1.2% (range 0.1% –3.2%) • Antisera collected from GDF9 immunized ewes to BMP15 – Averaged 1.8% (range 0.8%–3.0%). Results • Effect of Immunization on Ovulation Rate, Estrous Behavior Fertilization of Oocytes, Embryo Survival, and Lambing Percentages – Long term with peptide 1 • Ewes immunized with KLH alone maintained regular estrous cycles of 17 ± 2 days during the breeding season (10/10), whereas most ewes immunized against GDF9 (7/10) or BMP15 (9/10) failed to show regular estrus behavior, ovulation or cyclical progesterone profiles during the breeding season. – Long term with peptide 2 • Ewes in all treatment groups underwent normal cyclical estrus activity • No evidence either at laparoscopy (on two separate occasions) or at ovary recovery that any of these treatments impaired ovarian follicular activity. • Mean ovulation rates for the GDF9 and BMP15 groups were each significantly higher than that for the KLH group (both p < 0.01). • The respective mean ± S.E. ovulation rates were 1.6 ± 0.2 (N = 10), 3.6 ± 0.5 (N = 10) and 3.1 ± 0.4 (N = 9) for the KLH, KLH-GDF-9 peptide 2 and KLH-BMP-15 peptide 2 treatment groups, respectively. Results • Effect of Immunization on Ovulation Rate, Estrous Behavior Fertilization of Oocytes, Embryo Survival, and Lambing Percentages – Short term • Immunization against either GDF-9 or BMP-15 increased ovulation rate when compared to KLH immunized control ewes (P < 0.001) • The mean ovulation rates for the KLH, GDF9, and BMP15 immunized ewes were 1.8, 2.2, and 2.6, respectively. • Neither the ability of ovulated oocyte to be fertilized nor embryo survival was affected by immunization against either GDF9 or BMP15 • Furthermore, the ability of a ewe to carry her pregnancy to term was not affected by immunization against either GDF9 or BMP15 – Immunization did not affect the pattern of secretion and concentrations of progesterone during the estrous cycle and in pregnant immunized ewes. Conclusion • The identification of point mutations in the GDF9, BMP15 and ALK6 genes in sheep and BMP15 in humans, together with those from knockout mice have; provided new insights into how ovarian follicular development and ovulation rate are regulated in mammals as well as the importance of the oocyte in these processes. • Although sheep normally ovulate one or two eggs, the recently identified mutations in GDF9, BMP15 and ALK6 together with the additive effects of combination mutations in BMP15, GDF9 and ALK6 with or with- out FSH supplementation show that sheep are capable of ovulating between 0 and 23 under appropriate conditions. – In this context, sheep are, therefore, a powerful model in which to explore mechanisms controlling ovulation rate. Conclusion • These data suggest that – Secreted forms of both BMP-15 and GDF-9 are essential for follicular development and ovulation rate in sheep. – Immunization against either BMP-15 or GDF-9 peptides in long term with peptide 2 and short-term with peptide1, and not long term with peptide1 resulted in • Increased ovulation rate with no apparent detrimental effect on fertilization of released oocytes, ability of fertilized oocytes to undergo normal fetal development, or the ability of immunized ewes to carry a pregnancy to term. Conclusion • These data suggest that • Reasons why ? – Normal follicular development beyond the type 2 stage of growth did not occur and that phenotype was similar to that observed in ewes homozygous for mutations in BMP-15 or GDF-9. – Amino-acid sequences in peptide 1 correspond to flexible regions of the respective mature protein regions » and it is not known whether these regions are close to a binding site or a region important for dimerisation. – Neutralization of a large portion of GDF9 or BMP15 » Vs. partial immuno-neutralisation of GDF-9 or BMP-15 peptide in short term immunization. Conclusion • These data suggest that – Regulation of BMP15, GDF9, or both by immunisation with their peptides, demonstrate that by altering the bioavailability of GDF9 and BMP15 in vivo, is potentially a new technique for enhancing fecundity in some mammals; i.e. enhancing ovulation rate and increasing lamb production. – Oocyte appears to regulate the growth and differentiation of adjacent somatic cells • as well as their responsiveness to endocrine signals and thereby the number of follicles that mature and ovulate. – New possibilities for developing novel therapeutic reagents that targeting BMP-15, GDF-9 for regulating fertility in mammals. Conclusion • The increased ovulation rate observed in the heterozygous for mutations in BMP-15 or GDF-9 has been hypothesized to be • Dosage effect of BMP-15 or GDF-9 protein • Due to a dominant negative effect of the BMP-15 pro-peptide interfering with the formation of GDF-9 homodimers, BMP-15 homodimers, and/or GDF-9/BMP-15 heterodimers. – Because antibodies directed against the mature region of the protein are able to mimic the increased ovulation rate observed in heterozygous sheep, it seems likely that the increased ovulation rate observed in these ewes is the result of a decrease in the amount of active GDF-9 or BMP-15 protein available and not to any dominant negative actions of the GDF-9 or BMP-15 pro-region Conclusion • Future prospects – Twinning vaccines in sheep • Through the factors that regulate the follicle dominances. – Translating these findings in sheep to regulating ovulation rate and numbers of offspring in other animals, primates, wildlife or endangered species. Week points • The immunisation studies show that both GDF9 and BMP15 are essential for follicular development in sheep but they do not rule out the possibility that some of their effects are due to the presence of BMP15/GDF9 heterodimers. – Whether GDF9 and BMP15 act as heterodimers or separately to exert their effects through their receptors. • The Author did the research on Synchronized ewes – +ve: Synchronizations – -ve: The hormonal effects • This study was not designed to examine the effects of GDF9 or BMP15 immunization on increasing lamb production. – Such studies are underway and require much larger numbers of animals. – However, because no untoward effects were noted on pregnancy outcomes, there is a reasonable expectancy that immunization of sheep with GDF9 or BMP15 will lead to increased lamb production References, In order The End