* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Caput medusa sign
Survey
Document related concepts
Transcript
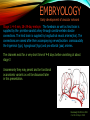
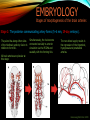
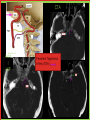
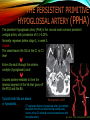
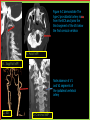
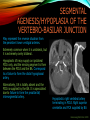
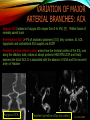
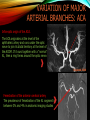
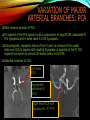
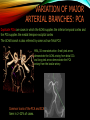
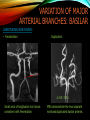
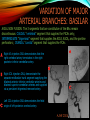
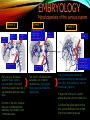
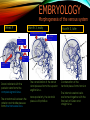
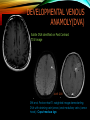
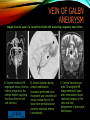
To Begin At The Beginning: Imaging Features And Embryological Basis Of Variants And Anomalies Of Cerebral Circulation eEdE-64 Komal Sharma MD , Betty Mathew MD , Thanuja Jeyakumar MD , Nishant Gupta MD , Joshua Sapire MD DISCLOSURE Komal Sharma, MD No Disclosures Betty Mathew, MD No Disclosures Thanuja Jeyakumar, MD No Disclosures Nishant Gupta, MD No Disclosures Joshua Sapire, MD No Disclosures EMBRYOLOGY Early development of vascular network 4 weeks 2-4 weeks: Initially the exposed neural plate and groove and the open neural tube are simply fed by diffusion from the amniotic fluid 5-8 weeks: After closure of the neural tube it is surrounded by a dense connective tissue, the meninx primitiva which contains meningeal vascular meshwork . Diffusion of nutrients to the neural tissue reach by diffusion from the peripheral meninx primitiva Cephalic portion of the neural tube grows and forms 3 primary brain vesicles, the meninx invaginates into the roofs of the prosencephalic and rhombencephalic vesicles, forming the primordia of the choroid plexuses (5–7 weeks). At this stage diffusion of nutrients to the neural tissue is both peripheral from the meninx primitiva and ventricular from the developing choroid plexuses 5-8 weeks Meninx primitiva The brain arteries evolve from the differentiation of specific choroid feeders within the meningeal vascular meshwork Neurosurg Clin N Am 2010 EMBRYOLOGY Early development of vascular network 5-8 weeks CONTD: Meninx primitiva begins to undergo the dramatic changes forming calvarium, dura, fluid-filled lepto-meninges, choroid plexuses and opening of the fourth ventricular outlets. Vascular meshwork follows this meningeal reorganization: The deeper vascular endothelium that covers the wall of the neural tube flattens and takes the appearance of a capillary network. The superficial vascular layer takes the appearance of larger and more continuous channels that form clear connections with the paired dorsal aorta and cardinal veins, and will eventually become the major brain arteries and veins. The communications between superficial and deep layers become the branches of the arteries and the tributaries of the veins Neurosurg Clin N Am 2010 EMBRYOLOGY Early development of vascular network Stage 1: 4–5 mm, 28–29-day embryo: The forebrain as well as hind brain is supplied by the primitive carotid artery through carotid-vertebro basilar connections. The hind brain is supplied by longitudinal neural arteries(lna). The connections are named after their accompanying nerves/location: craniocaudally the trigeminal (tga), hypoglossal (hga) and pro-atlantal (paa) arteries. The channels exist for a very short time of 4-8 days before vanishing at about stage 3 Uncommonly they may persist and be functional as anatomic variants as will be discussed later in this presentation. Neurosurg Clin N Am 2010 Contrib Embryol 1948 EMBRYOLOGY Stages of morphogenesis of the brain arteries Stage 2: The posterior communicating artery forms (5–6 mm, 29-day embryo). The paired lna along either sides of the hindbrain unite by fusion in midline to form BA . All brain arteries are plexular at this stage Simultaneously, the lna become connected cranially to anterior circulation via the PCOMs and caudally with the forming VAs The new blood supply results in the regression of the trigeminal, hypoglossal and proatlantal arteries. Neurosurg Clin N Am 2010 EMBRYOLOGY Stages of morphogenesis of the brain arteries Stage 3: The forebrain arteries can be recognized; the basilar and vertebral arteries are completed (7–12 mm, 32 days). The vertebral artery (VA) forms as a longitudinal paravertebral anastomosis between the intersegmental cervical arteries from C1 to C7 Developing choroid plexuses Stage 4: The mature pattern becomes apparent (12–14 mm, 35 days). Stage 5: The choroid stage, the adult pattern has become obvious (16–18 mm, 40-day embryo). The choroid plexuses are large, well vascularized structures loaded with glycogen and they strongly determine the prominence of their feeding arteries: ACA, ACHA, PCHA, from which the whole brain vasculature originates. Neurosurg Clin N Am 2010 EMBRYOLOGY Stages of morphogenesis of the brain arteries Stages 6: (20–24 mm, 44 days) and stage 7 (40 mm, 52 days), the mature pattern is completed with the circle of Willis and the capture by the posterior hemispheres of the vertebro-basilar blood supply. Neurosurg Clin N Am 2010 VARIATIONS OF INTRACRANIAL ARTERIES Persistent Vestigial Carotid-Vertebrobasilar Anastomoses Persistent primitive trigeminal artery (PPTA) Persistent primitive otic artery (PPOA) Persistent primitive hypoglossal artery (PPHA) Persistent primitive pro-atlantal intersegmental artery (PPIA) Segmental agenesis/hypoplasia of the vertebro-basilar junction Variations of the Leptomeningeal Segments of the Brain Arteries Variations of circle of Willis Variations of anterior cerebral artery Variations of posterior cerebral artery Variations of vertebrobasilar system Variations of middle cerebral artery PERSISTENT VESTIGIAL CAROTIDVERTEBROBASILAR ANASTOMOSES PERSISTENT VESTIGIAL CAROTID-VERTEBROBASILAR ANASTOMOSES Persistence of carotid and vertebral anastomosis is due to either a defect of the inhibition processes, or a defect of induction of the normally later-appearing vascular changes (eg, PCOM connection cranially and VA caudally). Persistent primitive trigeminal artery (PPTA) Persistent primitive otic artery (PPOA) Persistent primitive hypoglossal artery (PPHA) Persistent pro-atlantal intersegmental artery (PPIA) PERSISTENT PRIMITIVE TRIGEMINAL ARTERY (PPTA) PPTA is the most common vestigial artery observed with a reported pre-valance of 0.1-0.6% PPTA artery normally regresses at week 5, before stage 3 PPTA have been classified in different types depending on the territory they supply Saltzman type I is when it supplies the upper BA with the paired ASCAs and PCAs; the proximal BA is typically hypoplastic and the ipsilateral PCOM is missing. Saltzman type II is when it supplies the BAs with the ASCAs only, both PCAs being supplied by the ICAs through the PCOMs Acta Radiol 1959 CTA Raybaud, 2010 Axia l MIP Persistent Trigeminal Artery (TGA) CTA PERSISTENT PRIMITIVE TRIGEMINAL ARTERY (PPTA) Course: A PPTA arises from the cavernous ICA near the posterior genu, and may follow either a para- or an intra-sellar course. Ohshiro et al. has classified this into two types: Medial type : PTA runs through the dorsum sellae and perforates the dura near the clivus Lateral type : PTA runs between the sensory root of the trigeminal nerve and the lateral side of the sellae and penetrates the dura mater medial to Meckel’s cave Clinical Importance: May be injured in surgical procedures in the cavernous sinus or the posterior fossa. The PTA are also associated with increased prevalence of other vascular abnormalities such as aneurysms. Aneurysms are found in nearly 14% of all cases Neurosurgery 1993 THE PERSISTENT PRIMITIVE OTIC ARTERY (PPOA) The persistent otic artery (POA) is exceedingly rare. It is existence is questioned by many. Only 8 cases are reported in literature none of which convincingly displayed typical features. In case you see one one day!! To be a real otic artery: Artery should arise from the lateral most portion of the petrous segment of the ICA (proximal to the carotico-tympanic artery) Course internal auditory meatus Join the BAs at its caudal end. THE PERSISTENT PRIMITIVE HYPOGLOSSAL ARTERY (PPHA) The persistent hypoglossal artery (PHA) is the second most common persistent vestigial artery with prevalence of 0.1–0.25%. Normally regresses before stage 2, in week 5. Course: The vessel leaves the ICA at the C1 to C3 level Enters the skull through the anterior condylar (hypoglossal) canal Courses postero-medially to form the terminal segment of the VA that gives off the PICA and the BA. Typically both VAs are absent Radiographics 2009 or hypoplastic. CT angiogram depicts a hypoglossal artery (arrowhead) that arises from the proximal internal carotid artery (arrow) at the C2 vertebral level and anastomoses with the basilar artery. Ann Anat 1995, Radiographics 2009 THE PERSISTENT PROATLANTAL INTERSEGMENTAL ARTERY (PPIA) The persistent pro-atlantal intersegmental artery (PPIA), corresponds to the first spinal intersegmental artery. The PPIA disappears at stage 3, in week 6, when the VA becomes functional. Even in normal anatomy, it persists as the horizontal segment of the vertebral artery that passes between the occipital bone and C1, and as portions of the occipital artery. Classifications Type-1 proatlantal artery is a persistent primitive proatlantal artery. The type-1 proatlantal artery rises from the ICA or ECA and runs upward and dorsolaterally and joins the fourth segment of the VA. type-2 proatlantal artery is a persistent primitive first cervical intersegmental artery. The type-2 proatlantal artery rises from the ECA and joins the third segment of the VA below the first cervical vertebra Stroke 1993 Figure A-C demonstrate The type-2 pro-atlantal artery rises from the ECA and joins the third segment of the VA below the first cervical vertebra B. Axial MIP: A. Sagittal MIP: Note absence of V1 and V2 segments of the ipsilateral vertebral artery C. 3D D. Coronal MIP SEGMENTAL AGENESIS/HYPOPLASIA OF THE VERTEBROBASILAR JUNCTION SEGMENTAL AGENESIS/HYPOPLASIA OF THE VERTEBRO-BASILAR JUNCTION May represent the reverse situation from the persistent lower vestigial arteries. Extremely common when it is unilateral, but it is extremely rarely bilateral. Hypoplastic VA may supply an ipsilateral PICA only, and the missing segment is then between the PICA and the BA. Correspond to a failure to form the distal hypoglossal artery. Alternatively, VA is totally absent and the PICA is supplied by the BA. It is speculated due to failure to form the proatlantal/ intersegmental artery. Hypoplastic right vertebral artery terminating in PICA. Right superior cerebellar and PCA supplied by BA Neurosurg Clin N Am 2010 VARIATIONS OF THE LEPTOMENINGEAL SEGMENTS OF THE BRAIN ARTERIES CIRCLE OF WILLIS VARIATIONS Variations of circle of willis are extremely common The variations are thought to reflect hemodynamic balance of an individual. This balance may even change overtime and circle of wills may remodel accordingly Fenestrations reflect original plexiform arrangement and defined as division of the arterial lumen into distinctly separate channels, each with its own endothelial and muscularis layers, while the adventitia may be shared. Duplication is defined as two distinct arteries with separate origins and no distal arterial convergence. Hypoplasia of any segment is common. Complete aplasia rare. Complete aplasia certainly may compromise collateral flow in case of disease. Pergamon Press; 1963 VARIATION OF MAJOR ARTERIAL BRANCHES: ACA Azygous ACA (ncidence of azygos ACA ranges from 0 to 5%) [9] : Midline fusion of normally paired trunk Bi-hemispheric ACA (2–7% of anatomic specimens) (10): Very common. A1 ACA hypoplastic and contralateral ACA supplies via ACOM Persistent primitive olfactory artery arises from the terminal portion of the ICA, runs along the olfactory bulb, makes an abrupt posterior HAIR PIN LOOP, and finally becomes the distal ACA. It is associated with the absence of ACoA and the recurrent artery of Heubner Azygous ACA Persistent primitive olfactory artery Eur radiol 2002 VARIATION OF MAJOR ARTERIAL BRANCHES: ACA Infra-optic origin of the ACA. The ACA originates at the level of the ophthalmic artery and runs under the optic nerve to join its distal territory at the level of the ACOM. If it ours together with a ‘‘normal’’ A1, then a ring forms around the optic nerve. Raybond 2010 Fenestration of the anterior cerebral artery The prevalence of fenestration of the A1 segment is between 0% and 4% in anatomic imaging studies Fenestration VARIATION OF MAJOR ARTERIAL BRANCHES: MCA Duplicated MCA: Early origin of one MCA trunk from ICA Early vascular embryogenesis : Two forebrain arteries emerge from the ICA :the ACA and the ACHA. Both the artery of Heubner and the MCA develop as basal striatal branches of the primitive ACA. Typically, the cortical branches of the artery of Heubner supply the frontobasal cortex adjacent to the medial striatum, and the more proximal duplicate MCA supplies the temporal territory of the MCA Accessory MCA: MCA branch from ACA (also thought to be cortical extension of recurrent artery of heubner). Higher chances of aneurysm at accessory MCA origin. Radiographics 2009 VARIATION OF MAJOR ARTERIAL BRANCHES: ANTERIOR CHOROIDAL ARTERY (ACHA) Hyperplastic ACHA The anterior choroidal artery, usually a small ves sel, arises from the supraclinoid internal carotid artery just distal to the posterior communicating artery. From there it subdivides into important branches that supply the cerebral peduncle and optic tract. The temporo-occipital branches of the posterior cerebral artery may arise from the anterior choroidal artery is described as hyperplastic ANTERIOR CHOROIDAL ARTERY. The prevalence of hyperplastic anterior choroidal arteries reported to be 1.1%–2.3% Hyperplastic anterior choroidal artery (straight arrow),ipsilateral posterior communicating artery (arrow-head), and contralateral fetal posterior cerebral artery (curved arrow). VARIATION OF MAJOR ARTERIAL BRANCHES: PCA Most common variation of PCA. P2 segment of the PCA appears to be a continuation of large PCOM. Associated P1 PCA hypoplasia and in some cases A1 ACA hypoplasia. Embryologically, represents failure of the P comm (a remnant of the caudal embryonic ICA) to regress with resulting hypoplasia or agenesis of the P1 PCA segment that serves to connect the basilar artery to the PCA. Reported incidence of 22%. Left fetal PCA Hypoplastic ipsilateral A1 ACA Right fetal PCA with Hypoplastic P1 PCA VARIATION OF MAJOR ARTERIAL BRANCHES: PCA Duplicate PCAs are cases in which the ACHA supplies the inferior temporal cortex and the PCA supplies the medial temporo-occipital cortex The ACHA branch is also referred by some as true ‘fetal PCA’ MRA, 3D reconstartuction: Small pink arrow demonstrate the ACHA arising from distal ICA and long pink arrow demonstrate the PCA arising from the basilar artery. Common trunk of the PCA and SCA : Seen in 2–22% of cases. VARIATION OF MAJOR ARTERIAL BRANCHES: BASILAR LONGITUDNAL NON FUSION: • Fenestration Duplication AJNR: 2004 Small area of longitudnal non fusion consistent with fenestration MRA demonstrate the two separate nonfused duplicated basilar arteries. VARIATION OF MAJOR ARTERIAL BRANCHES: BASILAR AXIAL NON FUSION: The 3 segments that are constitutive of the BAs remain discontinuous: CAUDAL ‘‘vertebral’’ segment that supplies the PICAs only; INTERMEDIATE ‘‘trigeminal’’ segment that supplies the AICA, ASCA, and the pontine perforators ; CRANIAL ‘‘carotid’’ segment that supplies the PCAs Right VA injection DSA demonstrates that the right vertebral artery terminates in the right posterior inferior cerebellar artery. Right ICA injection DSA, demonstrate the separate midbasilar trunk segment supplying the bilateral anterior inferior cerebellar arteries and bilateral superior cerebellar arteries and supplied via a persistent trigeminal remnant artery. Left CCA injection DSA demonstrates the fetal origin of left posterior cerebral artery. AJNR 2004 DEVELOPMENT OF INTRACRANIAL VENOUS SYSTEM Before the intrinsic vasculature develops, the venous drainage is strictly meningeal and choroidal; transient vein of Markowski. 5 and 6 weeks : Vascular network is confined to the meninx primitiva. The pericerebral meshwork divides into : Deep (future pial) layer over the brain surface which is simple capillary layer. Superficial (future dura) layer become continuous and connect with the cardinal veins, to form early venous trunks. Connections between the superficial layer and the deep capillary layer early form of bridging veins. EMBRYOLOGY Morphogenesis of the venous system Week 6 ANTERIOR PLEXUS MIDDLE PLEXUS Week 7 POSTERIO PLEXUS PRIMARY HEAD SINUS Early venous drainage system. Three venous plexuses (ant, mid, post) drain the neural tube into a ventrolateral primary head sinus Growth of the otic capsule induces collateralization between the anterior and middle plexuses Collateralization between the anterior and middle plexuses Week 8 OTIC CAPSULE PRIMARY HEAD SINUS The dorsal collateralization develops via a lateral anastomosis. The primary head sinus becomes the jugular vein OTIC CAPSULE Collateralization between the posterior and middle plexuses The choroid plexus develops drained ventrally by diencephalic vein (dv) and dorsally by vein of Markowski (mpvm). The growth of the otic vesicle obliterates the primary head sinus Collateral develop dorsal to the otic vesicle between the middle and the posterior plexuses EMBRYOLOGY Morphogenesis of the venous system Month 3, early Week 9 Month 3, late SUPERIOR SAGITTAL SINUS DORSAL COLLATERAL TENTORIAL PLEXUS SIGMOID SINUS POSTERIOR STEM TRANSEVERSE SINUS Dorsal collateral with the posterior stem forms the complete sigmoid sinus. The anastomosis between the anterior and middle plexuses forms the transverse sinus. The consolidation of the dorsal dural plexuses forms the superior sagittal sinus . More posteriorly, the tentorial plexus is still primitive Condensation of the tentorial plexus forms torcular The internal cerebral veins are formed together with the final vein of Galen and straight sinus DEVELOPMENTAL VENOUS ANOMALY(DVA) Also known as cerebral venous angioma, DVA are common congenital variants of cerebral venous drainage that are frequently identified in imaging studies. They are most common cerebral vascular malformations , accounting for ~55% of all such lesions. Usually incidentally found with no consequence to the patient. The brain surrounding the DVA is nearly always normal, and histologically the anomaly is limited to the venous structures, without involvement of capillaries or arteries. Imaging appearance: Caput medusae sign. Veins draining into a single larger collecting vein, which in turn drains into either dural sinus or into a deep ependymal vein. DEVELOPMENTAL VENOUS ANAMOLY(DVA) Subtle DVA identified on Post Contrast T1W image AJNR 2008 SWI and, Postcontrast T1-weighted image demostarting DVA with draining vein (arrow) and medullary veins (arrow head) : Caput medusa sign VEIN OF GALEN ANEURYSM Developmental Theory: Raybaud proposes that vein of Galen aneurysms are not a result of dilatation of the veins of Galen, but rather a consequence of dilatation of persistent median prosencephalic veins. The evidence he cites supporting this statement includes the following: (a) The vein of Galen develops late and lacks connections to the choroidal branch of the anterior cerebral artery, which is a primary feeder in most vein of Galen aneurysms; (b) The typical vein of Galen aneurysm directly drains both prosencephalic and mesencephalic arteries in a pattern typical of the median prosencephalic vein, but the normal mature vein of Galen does not. (c) Anomalous venous drainage as described in previous sections probably represents persistent fetal drainage that remains intact because it effectively deals with the high-flow system. Such persistent fetal drainage may prevent the development of the normal sinus system. Neurosurg Clin N Am 2010 VEIN OF GALEN ANEURYSM Images from the case of a 3-month-old triplet with worsening congestive heart failure. A. Volume-rendered MR B, Carotid injection during angiogram shows, from an arterial embolization inferior prospective, the procedure performed when arterial feeders supplying the patient was 6 months old the lesion from the left shows residual flow to the side (arrows). lesion from pericallosal and Blaise V. Jones et al. AJNR Am J Neuroradiol 2002;23:1717-1724 posterior choroidal arteries (arrowheads). ©2002 by American Society of Neuroradiology C, Coronal view fast spinecho T2-weighted MR image obtained 6 years after embolization shows continued patency of the varix and mild enlargement of extra-axial fluid spaces. REFERENCES 1. Raybaud. Normal and Abnormal Embryology and Development of the Intracranial Vascular System. Neurosurg Clin N Am 2010; 21: 399–42 2. Padget DH. The development of the cranial arteries in the human embryo. Contrib Embryol ;1948: 212(32):205–271 3. Saltzman G. Patent primitive trigeminal artery studied by cerebral angiography. Acta Radiol 1959;51: 329–336 4. Ohshiro S, Inoue T, Hamada Y, et al. Branches of the persistent primitive trigeminal artery: an autopsy case. Neurosurgery 1993; 32:144–147 5. Caro R de, Parenti A, Munari PF. The persistent primitive hypoglossal artery: a rare anatomic variation with frequent clinical implications. Ann Anat 1995;177:193–198 6. Dimmick S, Faulder K .Normal Variants of the Cerebral Circulation at Multidetector CT Angiography RadioGraphics 2009; 29:1027–1043. REFERENCES 7. Bahsi YZ, Uysal H, Peker S et al. Persistent primitive proatlantal intersegmental artery (proatlantal type 1) results in ‘‘top of the basilar” syndrome. Stroke 1993;24:2114–2117 8. Klosovskii BN. Fundamental facts concerning the stages and principles of development of the brain and its response to noxious agents. London Pergamon Press 1963; 3–43. 9. Calzolari F, Ceruti S, Pinna L et al. Aneurysm of the azygos pericallosal artery. J Neurosurg 1991;18:277– 285 10. Perlmutter D, Rhoton AL. Microsurgical anatomy of the distal anterior cerebral artery. J Neurosurg.1978;49(2):204-28. 11. Y. Fushima, Y. Mikib, K. Togashib et al. A Developmental Venous Anomaly Presenting Atypical Findings on Susceptibility-Weighted Imaging AJNR August 2008; 29: e56.