* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Measurement of charge to mass ratio on an electron
Magnetic monopole wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Lorentz force wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Electromagnet wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Superconductivity wikipedia , lookup
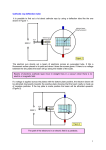
Measurement of charge to mass ratio on an electron R.L.Griffith,A.Okunyan,A.Okunyan,M.R.Levi ABSTRACT The charge to mass ratio of an electron was studied using an electron beam tube and two Helmholtz coils. The set up of the apparatus is included with this report. This is the same experiment that was conducted by J.J. Thompson to measure the charge to mass ratio in the early 20th century. A beam of electrons was accelerated through a known potential difference, and passed through a magnetic field causing the electrons to move in a circular orbit. The radius of curvature was calculated with placing the beam through a known distance marker and the voltage and current were measured at those markers. The error calculations yielded a value of 8.6 %. The errors acquired were within the allowed errors of these experiment. Subject headings: Magnetic Force, Magnetic field, Electron 1. Introduction rewriting equation 4 we get 2V e = 2 2 2 m G I r When a cathode electrode is heated, it begins to emit a large number of electrons. These electrons are then accelerated through a potential difference V to a velocity v. A hole in the anode allows the electrons to form a beam of electrons who are than focused ny a Wehnelt cylinder to which a negative voltage is applied to produce a well defined beam. As the electrons move perpendicular to a magnetic field produced by the Helholtz coil they are deflected by the Lorentz force Fl = evB (1) where Z is Z= therefore 2. 2.1. (2) the kinetic energy is given by K= mv2 = ev 2 (3) 2.2. combining equations 2 and 3 we can find an equation for the charge to mass ratio given by 2V e = 2 2 m B r (8) e Z = 2 m G (9) Method Equipment Used Equipment Electron beam tube Helmholtz coils Battery eliminator Variac magnetic compass Variable power supply Voltmeter thus the electrons moves along a circular path of radius r such that mv2 evB = r 2V (Ir)2 (7) Serial n/a n/a n/a n/a n/a n/a Procedure The first step is to set up the equipment as outlined in the included diagram. Next all the equipment is turned on and the electron beam is then focused using the focusing voltage. The current regulator is then adjusted to increase the magnetic field that will cause the electron beam to move in a circular fashion. there are four measurements taken, with the beam at different radius. The voltage is increased by increments of 50 V and four different trial are performed with the same voltages. (4) The magnitude of the magnetic field is found for the geometry of the Helmholtz coils and the field current I by B = GI (5) where G is the geometric factor for the coils given by G = 2.48 × 10− 4T /A(±2%) (6) 1 3. Results and Discussion an error estimate was performed on this lab to compare the experimental values obtained during this experiment and the theoretical values obtained from the actual mass and charge of an electron. Some possible sources of error can be explained due to the precision of the measurements taken by the instruments used, i.e voltmeter and amp-meter. Another source of error can be explained by the measurement of the radius of the beam, due to accuracy of the radius. The error measurement yielded a value of 8.53 % which confirms the validity of the principles used to conduct this experiment. There are five calculations performed, the first one is to find the value of Z, which is performed using equation 8. Next the average of Z is calculated by taking the sum of all the values and deviding by the total number of Z’s. Next the charge to mass ratio is calculated for the experimental value by using equation 9 and is then compared to the theoretical value that is calculated by using the theoretical values of the charge of an electron which is 1.60×10−19 and the value of the mass of an electron which is 9.109×10−31 . The calculated value for the error measurements yielded a value of 8.57 %, which is within the range allowed due to errors introduced by taking measurements. 3.1. Acknowledgements The author would like to thank Roni,Alina,Arman, and Simon for their help with this experiment. Data and Calculations radius Acce. voltage Current Z V (cm) V(V) I(A) (Am)2 250 V 2 247.9 3.71 9.02 × 104 3 246.8 2.43 9.29 × 104 4 246.9 1.80 9.53 × 104 5 247.7 1.53 8.47 × 104 300 V 2 301.7 4.02 9.33 × 104 3 301.3 2.71 9.12 × 104 4 301.0 2.05 8.96 × 104 5 300.6 1.68 8.52 × 104 350 V 2 350.1 4.45 8.84 × 104 3 349.7 2.87 9.43 × 104 4 348.9 2.20 9.01 × 104 5 349.3 1.82 8.44 × 104 400 V 2 401.0 4.65 9.27 × 104 3 402.0 3.12 9.18 × 104 4 402.0 2.36 9.02 × 104 5 401.0 1.95 8.35 × 104 Z average V 8.98 × 104 (Am) 2 Experimental and theoretical values for C Exp. 1.60 × 1011 kg 11 C Theo. 1.76 × 10 kg Error 8.57 % 4. 5. REFERENCES Los Angeles City College Lab Manual Physics 102. e m Conclusion This lab was conducted to get a better understanding on the effects that a magnetic field has on a charged particle. It allows us to verify the principles of electricity and magnetism. This 2-column preprint was prepared with the AAS LATEX macros v5.2. 2