* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Equipartition theorem wikipedia , lookup
Dynamic insulation wikipedia , lookup
Thermal radiation wikipedia , lookup
Heat exchanger wikipedia , lookup
Conservation of energy wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Calorimetry wikipedia , lookup
Heat capacity wikipedia , lookup
R-value (insulation) wikipedia , lookup
Temperature wikipedia , lookup
Countercurrent exchange wikipedia , lookup
Extremal principles in non-equilibrium thermodynamics wikipedia , lookup
Thermoregulation wikipedia , lookup
Heat equation wikipedia , lookup
Internal energy wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Heat transfer wikipedia , lookup
Heat transfer physics wikipedia , lookup
Thermal conduction wikipedia , lookup
Thermodynamic system wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Hyperthermia wikipedia , lookup
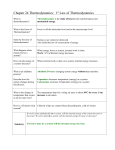
1 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 1 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Moving 2011 Life Towards 2012 COME FOR SUCCESS CONTAIN: 1. THERMODYNAMICS. 2. THERMODYNAMIC SYSTEM AND KINDS 3. FIRST LAW OF THERMODYNAMICS. 4. APPLICATIONS OF FIRST LAW OF THEMODYNAMICS. 5. CP - CV = R 6. SECOMD LAW OF THERMODYNAMICS 7. CARNOT ENGINE. 8. ENTROPY 9. EQUATIONS 10. DIMENSIONS 11. SHORT QUESTIONS AND ANSWERS Thermodynamics Introduction: Thermodynamics is a branch of physics which deals with the energy and work of a system. Thermodynamics deals only with the large scale response of a system which we can observe and measure in experiments. Gases have various properties that we can observe with our senses, including the gas pressure p, temperature T, mass n, and volume V that contains the gas. Careful, scientific observation has determined that these variables are related to one another, and the values of these properties determine the state of the gas. A thermodynamic process, such as heating or compressing the gas, changes the values of the state variables in a manner which is described by the laws of thermodynamics. The work done by a gas and the heat transferred to a gas depend on the beginning and ending states of the gas and on the process used to change the state. A thermodynamic system is a collection of matter and energy which has a distinct boundary. Matter or energy (heat) can be or can not be transferred across the system boundary. A thermodynamic system can be defined as macroscopic region of the universe, often called a physical system. Closed system: A closed system is one where energy can cross the boundary, but matter cannot. eg a sealed test tube. Isolated system: An isolated system is one where neither matter nor energy can cross between the system and the surroundings. The universe itself is an isolated system. Open system: Second YEAR GROUP TIME ------------- GROUP NO:- - - - -- - - - CHAPTER 11 HEAT 1 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #1 PTCL # 022-2670019 2 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 2 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Moving 2011 Life Towards 2012 COME FOR SUCCESS An open system is one where both matter and energy can freely cross from the system to the surroundings and back. eg an open test tube. Introduction: The first law of thermodynamics was formulated by Mayer; a much clearer formulation was provided by H. von Helmholtz in 1847. The First Law of Thermodynamics deals the energy is always conserved, it cannot be created nor destroyed. The energy can be converted from one form into another. Statement: A change in the internal energy of a closed thermodynamic system is equal to the difference between the heat supplied to the system and the amount of work done by the system on its surroundings. ΔU = ΔQ ΔW change in internal energy heat added to the system work done by the system Description: The first law of thermodynamics provides the existence of a state variable for a system, the U internal energy, and tells how it changes in thermodynamic processes. In this law given internal energy of a system to be reached by any combination of Q heat absorbed or rejected and work. It is important that internal energy is a variable of state of the system whereas heat and work change the state of the system. The change in the internal energy of a closed thermodynamic system is equal to the difference between the work done on the system and the amount of heat energy rejected by the system. ΔU = ΔW ΔQ change in internal energy work done on the system heat subtracted to the system If heat flows into a system or the surroundings to do work on it, the internal energy increases and the Q or W is positive. Conversely, heat flow out of the system or work done by the system will be at the expense of the internal energy, and will therefore be negative. There are a number of different thermodynamic processes are as follows: 1. Isobaric Process - The process in which “Pressure” is kept constant. The quantity of gas placed in a closed system by means of a moveable piston. Weights placed on top of the piston exert a force F over the cross-section area A, producing a Second YEAR GROUP TIME ------------- GROUP NO:- - - - -- - - - CHAPTER 11 HEAT 2 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #2 PTCL # 022-2670019 3 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 3 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Moving 2011 Life Towards 2012 COME FOR SUCCESS F , which is exactly countered by the pressure of the gas, so that the piston remains A stationary. Now suppose that we heat the gas by supplying heat Q ; the gas is allowed to expand, so the piston will be forced upward by a distance ΔY. Since this motion is opposed by the force, a quantity of work F ΔY will be done by the gas on the piston. The work done by the system on the surroundings is negative, so the work is given by: W = F Y W = P A Y W = P V According First law of thermodynamics Q = U + W Q = U + [P V ] Q =U + P V 2. Isochoric Process – The process in which “Volume” kept constant is also called an isometric process. The quantity of gas placed in a closed system by means of with fixed walls. And fixed piston. A gas enclosed in it, by adding ΔQ heat that will increase the internal energy ΔU. Due to increase of internal energy, the work done is zero in an isochoric process, say, ΔW = 0 by the system. According first law of thermodynamics, Mathematical Derivation: U= Q + W ΔU = Q + 0 ΔU = ΔQ. This equation is called Isochoric equation. 3. Isothermal Process- The process in which “Temperature” is kept constant. The quantity of gas placed in a closed system by means of a moveable piston. The system is placed over a hot body reservoir and the pressure on the system is decreased. Due to expansion, the temperature of the system is decreased but at the same time Q amount of heat is absorbed from the hot body reservoir and the temperature of the system is again maintained T = 0 The internal energy of an ideal gas is proportional to the temperature, so if the temperature is kept fixed the internal energy does not change. - U= - Q + W 0 = - Q + W Q = W 4. Adiabatic - In an adiabatic process, no heat is added or removed from the system. The quantity of gas placed in a closed system by means pressure P = Second YEAR GROUP TIME ------------- GROUP NO:- - - - -- - - - CHAPTER 11 HEAT 3 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #3 PTCL # 022-2670019 4 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 4 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Moving 2011 Life Towards 2012 COME FOR SUCCESS of a moveable piston. The system is placed over an insulator. When a gas expands, it does work on the surroundings; compression of a gas to a smaller volume similarly requires that the surroundings perform work on the gas. If the gas is thermally isolated from the surroundings, then the process is said to occur adiabatically. In an adiabatic change, Q = 0, so the First Law becomes U= Q + W The heat capacity at constant pressure of a gas is the amount of heat required to change the temperature of a unit-mass of gas one temperature degree at constant pressure. ΔQ p = c p n ΔT The heat capacity at constant pressure of a gas is the amount of heat required to change the temperature of a unit-mass of gas one temperature degree at constant volume. ΔQv = c v n ΔT No external work is being done when a gas is heated at constant volume i.e. gas uses all the heat which is given to it for increasing its internal energy. Hence if temperature of one mole of a gas is raised through 1oC, the molar heat capacity is given itself at constant volume by increase in internal energy. ΔQv = c v n ΔT But when a gas is heated at constant pressure there will be expansion of gas i.e. increase in volume take place and some external work will b done. For this some extra heat is required which ΔQ p = c p n ΔT should be given to the gas to perform the external work. ΔQ p > ΔQ v cp n ΔT > c v n ΔT Hence the Molar Heat capacity of a gas at constant pressure must be greater than Molar Heat capacity of a gas at constant volume. CP > CV Hence shown Second YEAR GROUP TIME ------------- GROUP NO:- - - - -- - - - CHAPTER 11 HEAT 4 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #4 PTCL # 022-2670019 5 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 5 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Moving 2011 Life Towards 2012 COME FOR SUCCESS Mathematical and Descriptive Proof: Suppose “n” moles gas is heat up while keeping its volume constant. The result of adding heat to the system is an increase of its T temperature. ΔQv = c v n ΔT Here, CV is the molar heat capacity at constant volume, ∆QV is the heat added, and ∆T is the resulting increase in the temperature of the system. The first law of thermodynamics shows that, ΔU = ΔQ - ΔW = cv n ΔT - ΔW Since the volume is kept constant (∆W = 0) we conclude that, ΔU = c v n ΔT Again heat is added to the system, the volume is changed such that the gas pressure does not change. The change in the internal energy of the system is given by first law of thermodynamics, ΔU = ΔQ - ΔW = c p n ΔT - ΔW where cp is the molar heat capacity at constant pressure. This expression can be rewritten as, ΔU + ΔW = cp n ΔT c v n ΔT + ΔW = c p n ΔT c v n ΔT + P(V2 -V1 ) = c p n ΔT c v n ΔT + P V = cp n ΔT For an ideal gas PV = n R T, we can relate ∆V to ∆T, if we assume a constant pressure P V = n R T c v n ΔT + n R T = cp n ΔT n ΔT cv + R = cp n ΔT cv + R = cp Or R= cp - c v we see that Cp ≠ CV. This shows that, difference between molar heat capacity at constant pressure and c at constant volume is equal to the universal gas constant. The values for γ are γ = p =1.4 for diatomic cv c 5 gasses like air and its major components, and γ = p = for monatomic gasses like the noble gasses. cv 3 3 5 The formulas for specific heats would reduce in these special cases: Monatomic: c v = R and c p = R 2 2 5 7 and for Diatomic: c v = R and c p = R 2 2 Second YEAR GROUP TIME ------------- GROUP NO:- - - - -- - - - CHAPTER 11 HEAT 5 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #5 PTCL # 022-2670019 6 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 6 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Moving 2011 Life Towards 2012 COME FOR SUCCESS The 1st Law does not tell us everything we need to know about thermodynamic processes. We need to develop an understanding of the 2nd Law of Thermodynamics in order to address issues relating to spontaneity, equilibrium and efficiency. There are two statements of this law: Clausius statement: The Clausius statement expresses as follows: No process is possible whose sole result is the transfer of heat from a body of lower temperature to a body of higher temperature. Description: Spontaneously, heat cannot flow from cold regions to hot regions without external work being performed on the system, for example. In a refrigerator, heat flows from cold to hot, but only when forced by an external agent, a compressor. Kelvin statement The Kelvin statement expresses as follows: No process is possible in which the sole result is the absorption of heat from a reservoir and its complete conversion into work. Description: This means it is impossible to extract energy by heat from a High-temperature energy source and then convert all of the energy into work. At least some of the energy must be passed on to heat a low-temperature energy sink. Equivalence of Kelvin’s and Clausius Statements: Description: In order to show the equivalence between two statements, it is supposed an ideal heat engine which can convert heat energy absorbed from high temperature reservoir into work without rejecting heat in to cold body reservoir. Thus Kelvin’s statement is false. Such ideal heat engine can combine, with an eclectic refrigerator so that the work produced by the engine, is used by the refrigerator. Now, the combined system is a heat engine and refrigerator which uses no external work, violating the Clausius statement of the second law. Thus we see that, the Kelvin and -Clausius statements are equivalent, and one necessarily implies the other. Heat engine Definition: The engine has minimum heat losses, and friction with maximum efficiency than all other heat engines, designed by French scientist Sadi Carnot, is called “Carnot Engine”. The carnot heat engine Second YEAR GROUP TIME ------------- GROUP NO:- - - - -- - - - CHAPTER 11 HEAT 6 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #6 PTCL # 022-2670019 7 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 7 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Moving 2011 Life Towards 2012 COME FOR SUCCESS (the ideal imaginary heat engine) Construction: A Carnot engine consists a cylinder with fixed distinct non- conducting boundaries, provided frictionless non-conducting piston. An ideal gas is kept init as the working substance. It operates under the control of two heat reservoirs one of them at high temperature T1 called source, while the other at lower temperature T2 called sink. The base of the system is conducting; it works in a cycle called “Carnot cycle”. Carnot Cycle: A cycle occurs when a system is taken through a series of different states and finally to its initial state. In the process of going through this cycle, the system may perform work on its surroundings. It consists of four basic reversible processes meaning that the cycle as a whole is also reversible. Such cycle, is known as “Carnot cycle. Operational Process: The Carnot cycle when acting as a heat engine consists of the following steps: Reversible isothermal expansion of the gas at the "hot" temperature, TH The heat source at high temperature T1 is contact with the cylinder which lowers the pressure in the gas by expansion. The temperature remains constant, but the volume increases. The expanding gas makes the piston work on the surroundings. The gas expansion is propelled by absorption of quantity Q1 of heat from the high temperature reservoir. Reversible adiabatic expansion of the gas (isentropic work output). The piston and cylinder are to be thermally insulated, thus a system can not gain heat. The gas continues to expand, working on the surroundings. The temperature decreases from TH to TC and the volume increases as the gas expands to fill the volume. The gas expansion causes it to cool to the "cold" temperature, TC. Reversible isothermal compression of the gas at the "cold" temperature, TC. (Isothermal heat rejection) Now the surroundings do work on the gas, causing quantity Q2 of heat to flow out of the gas to the low temperature reservoir. The sink at low temperature TC is brought into contact with the cylinder, which raises the pressure in the gas. The temperature remains constant, but the volume decreases. Reversible adiabatic compression of the gas (isentropic work input). Once again the piston and cylinder are to be thermally insulated. During this step, the surroundings do work on the gas. The temperature increases TC to TH and the volume decreases as the gas is compressed and it causing the temperature to rise to TH. Second YEAR GROUP TIME ------------- GROUP NO:- - - - -- - - - CHAPTER 11 HEAT 7 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #7 PTCL # 022-2670019 8 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 8 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Moving 2011 Life Towards 2012 COME FOR SUCCESS Efficiency: For a heat engine, the efficiency is the ratio of useful work performed to the heat energy consumed from the high-temperature reservoir Explanation: Many heat engines converting part of the heat energy into useful work. There are losses due to friction. Consider the example of a heat engine, which extracts heat energy Q1 from the combustion at temperature TH, and which expels some heat, Q2, into the exhaust at temperature TC. By the conservation of energy, the difference (Q1 – Q2) is the amount of Out Put work (mechanical energy) done. Efficiency = In put Useful work η= Heat energy expel Q - Q η= 1 2 Q1 Q η = 1 - 2 Q1 Thus the maximum efficiency of an ideal engine is controlled by the source temperature TH, which should be as high as possible, and the exhaust (sink) temperature TC, which should be as low as possible. A real engine will be even less efficient because of internal friction, and other factors. T Efficiency always measured in percentage. η = 1 - C TH "Entropy" is defined as a measure of unusable energy within a closed or isolated system (the universe for example). As usable energy decreases and unusable energy increases, "entropy" increases. Entropy is also a gauge of randomness or chaos within a closed system. As usable energy is irretrievably lost, disorganization, randomness and chaos increase .Thus entropy is the change of heat energy per maintained temperature. Description: The concept of entropy is defined by the second law of thermodynamics, which states that the entropy of an isolated system always increases or remains constant. Thus, entropy is also a measure of the tendency of a process, such as a chemical reaction, to be entropically favored, or to proceed in a particular direction. It determines that thermal energy always flows spontaneously from regions of higher temperature to regions of lower temperature, in the form of heat. These processes reduce the state of order of the initial systems, and therefore entropy is an expression of disorder or randomness. Mathematical derivation: Second YEAR GROUP TIME ------------- GROUP NO:- - - - -- - - - CHAPTER 11 HEAT 8 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #8 PTCL # 022-2670019 9 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 9 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Moving 2011 Life Towards 2012 COME FOR SUCCESS Suppose a very small quantity of heat Q is added slowly to a system at fixed maintained temperature T, then the entropy “s” of the system is increased by ΔQ , Δs = T Its unit is J K-1. The entropy will be decreased by the same amount if the same quantity of heat were removed at that that same temperature. Entropy, like energy, is an additive quantity, but unlike energy, entropy is not a conserved quantity. 1. Q = U + W 2. Q = U 3. ΔQ = ΔU + P ΔV 5. - ΔU = ΔW 6. - ΔU = -ΔW 7. PΔV = ΔW 9. cp - c v = k N A 10. Work done = Q1 - Q2 12. DIMENSION M L2 T -2 M L2 T -2 Molar heat capacity at constant Volume cv and at Pressure cp Internal energy increases or decreases UNIT J U J M L2 T -2 K 1 M L2 T -2 K W Temperature Second YEAR 13. ΔQ 14. Δs = T PHYSICAL QUANTITY & SYMBOL Heat energy supplied or rejected Q T Pressure 8. cp - c v = R work done 11. Eff.= 100 Q 1 Q Eff.= 1- 2 100 Q1 T Eff.= 1 - 2 100 T1 Work done 4. ΔQ = ΔW J(mol-1 K-1) J K M T -2 P GROUP TIME ------------- GROUP NO:- - - - -- - - - N/m2 CHAPTER 11 HEAT 9 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #9 PTCL # 022-2670019 10 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 10 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Volume Moving 2011 Life Towards 2012 L3 V m3 Universal Gas Constant R J(mol-1 K-1) Area M L2 T -2 K -1 L2 A m2 Entropy COME FOR SUCCESS s Efficiency Avogadro’s number NA M L2 T -2 K 1 Dimensionless Dimensionless J K-1 no unit per mol Q: No.1 What is Perpetual-Motion Machines. Answer: Any process will not occur unless it satisfies both the first and the second laws of thermodynamics. A lot of efforts were spent to create machines which violate the first law or the second law. This kind of machines is called perpetual-motion machines. Devices that violate the first law are called perpetual-motion machines of the first kind. Devices that violate the second law are called perpetual-motion machines of the second kind Q: No.2 Define First law of thermodynamics in terms of entropy? Answer The first law of thermodynamics is also called the Law of Conservation of Energy. This law suggests that energy can be transferred from one system to another in many forms. Also, it can not be created or destroyed. Thus, the total amount of energy available in the Universe is constant. Q: No.3 What are the first and second laws of thermodynamics? Answer: For polyatomic gas, First Law of Thermodynamics: Conservation of Energy Second Law of Thermodynamics: Entropy increases in all natural processes Q: No.4 Why heating produced adiabatic compression? Answer: An adiabatic is a process in which, heat energy neither enters nor leaves the system. When a system is compressed, the work is to be done on the system. The enclosed gas molecules become more energetic and heating produced. Such heat energy can’t leave the system. Hence, "heating produced Second YEAR GROUP TIME ------------- GROUP NO:- - - - -- - - - CHAPTER 11 HEAT 10 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #10 PTCL # 022-2670019 11 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 11 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Moving 2011 Life Towards 2012 COME FOR SUCCESS adiabatic compression”. Q: No.5 Why adiabatic process is steeper than isotherm? Answer: In the adiabatic process the thermodynamic variables are temperature, volume and pressure. And in the isothermal process volume and pressure are the variables at constant temperature. So that, the graph determined in adiabatic is steeper than isotherm. Q: No.6 Define second law of thermodynamics in terms of entropy? Answer: The second law can be thought of as a qualitative law: the second law says that all natural processes occur in such a way as to result in an increase in entropy. To understand this law, it is first necessary to explain the concept of entropy. Entropy means disorder. Consider the dissolving of a sugar cube in water. The sugar cube itself represents a highly ordered state in which every sugar particle is arranged in an exact position within the sugar crystal. The entropy of a sugar cube is low because there is little disorder. But consider what happens when the sugar cube is dissolved in water. The cube breaks apart, and sugar molecules are dispersed completely throughout the water. There is no longer any order among the sugar molecules at all. The entropy of the system has increased because the sugar molecules have become completely disorganized Q: No.7 Entropy has often been called as “time’s arrow”. Explain? Answer The second law of thermodynamics is that it tells us in which direction processes go. The processes in which order increases or entropy decreases, this process not happen in our daily life. Hence, entropy has been called “times arrow”, for it tells in which direction time is going. Q: No.8 Under what condition, is it possible for the entropy of system to decreases? Answer: When heat is removed from the system, the heat energy is negative. Thus, entropy of the system decreases. The example is ice. When water is cooled at constant temperature, the heat is to be extracted. Hence, entropy decreases. Q: No.9 What are some factors that affect the efficiency of automobile engines? Answer: The efficiency of automobile engine depends onto the hot body and cold body temperature T reservoirs. If the ratio C is greater than efficiency will be smaller or reverse. TH Q: No.10 Is it possible, according to second law of thermodynamics to construct an engine that will be free from “thermal pollution”? Answer: No, it is not possible to construct an engine, which will be free from, thermal pollution. We can Second YEAR GROUP TIME ------------- GROUP NO:- - - - -- - - - CHAPTER 11 HEAT 11 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #11 PTCL # 022-2670019 12 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 12 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Moving 2011 Life Towards 2012 COME FOR SUCCESS construct an engine, which minimize the thermal pollution. Q: No.11 Can a given amount of mechanical energy is converted completely in to energy? If so, give an example. Answer: Yes, it is possible that a given amount of mechanical energy be converted completely in to heat energy. The example of such statement is ideal heat engine. According to the first law of thermodynamics, Q = U+ W Q = 0 + W Or, Q = W Q: No.12 What is meant by heat death of universe? Answer: The 'heat-death' of the universe is when the universe has reached a state of maximum entropy. This happens when all available energy (such as from a hot source) has moved to places of less energy (such as a colder source). Once this has happened, no more work can be extracted from the universe. Since heat ceases to flow, no more work can be acquired from heat transfer. This same kind of equilibrium state will also happen with all other forms of energy. Since no more work can be extracted from the universe at that point, it is effectively dead, especially for the purposes of humankind. Q: No.13 Name a process in which volume remains constant? Answer: The name of process, in which volume of a system remains constant, is “Isochoric process”. Q: No.14 Name a process in which heat energy is transferred to or from the system but the temperature of the system does not change. Answer: The name of a process in which heat is transferred to or from the system but the temperature of the system remains constant is called “isothermal process”. Q: No.15 Why, does the temperature drop in adiabatic expansion process? Answer: When a gas in isolated system is to be heated, heat energy doesn’t leave the system. The work is to be done at the cost of internal energy. So that temperature of the system drops. Q: No.16 A gas expands adiabatically. Does the gas perform any work? What is the source of energy needed to do this work? Answer: When a gas expands adiabatically. Yes, the gas performs work. The source of energy, to do work is internal energy. Q: No.17 A thermos flask contains milk. The flask is shaken rapidly. Consider the milk as the system. Does the temperature of milk rise? Has heat been added to it? Has work been done on it? Has its internal energy changed? Answer: Second YEAR GROUP TIME ------------- GROUP NO:- - - - -- - - - CHAPTER 11 HEAT 12 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #12 PTCL # 022-2670019 13 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 13 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Moving 2011 Life Towards 2012 COME FOR SUCCESS A thermos flask contains milk. The milk is shaken rapidly. Consider the milk as the system. Yes, the temperature of milk rises. No, heat has not been added to it. Yes, work has to be done on it. Yes, internal energy of the system increases. Q: No.18 A gas is allowed to expand at constant temperature. It does external work. Does the internal energy of the gas change during this process? If not, what is the source of energy needed to do this work? Answer: A gas is allowed to expand at constant temperature, cording to the application say isothermal process of first law of thermodynamics. It does external work during the process. No, the temperature of the gas does not change during this process. The source of energy, needed to do this work is heat reservoir. We know that, Q = U +W Q = 0 + W Or, Q = W Q: No. 19 Comment the statement the heat engine covert disorder motion in to order motion. Answer: We know that heat engine operates in a cycle, it absorbs heat from hot body, coverts some of it into work and part of energy rejects to cold body. When the heat engine works, it receives the heat from hot body, which appears in disordered motion. This heat energy is converted in to mechanical work by heat engine. It means, disordered motion is converted in to ordered motion. As we have knowledge that heat increases the, disorder of molecules. When heat coverts work, the disordered motion also converts in to ordered motion. Q: No. 20 Why more work is to be done, when a gas is heated at constant pressure than at constant volume? Answer: When gas is to be heated at constant volume then all the heat energy supplied to the system is to be used to increase internal energy. Hence no work is to be done. Q = U + W Or, Q = U (say isochoric process) And gas is to be heated at constant pressure then part of heat energy is used to increase the internal energy and reaming part is used to perform external work. Q = U + W Q = U + PV (say isobaric process) This show that more energy is to be required at constant pressure than at constant volume. Q: No.21 Write the formula of entropy? Answer Q S = T Q: No.22 Give an example of a process in which no heat transferred to or from the system but the temperature of the system changes. Second YEAR GROUP TIME ------------- GROUP NO:- - - - -- - - - CHAPTER 11 HEAT 13 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #13 PTCL # 022-2670019 14 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 14 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Moving 2011 Life Towards 2012 COME FOR SUCCESS Answer: A process in the thermodynamics is adiabatic process, in which no heat enters or leaves the system. During this process if we decrease the pressure on the system, the volume increases i.e. expansion occurs and work is done by the internal energy of the system. Due to decrease in internal energy hence, temperature falls. Q: No.23 Is it possible to construct a heat engine that will not expel heat into atmosphere? Answer: According second law of thermodynamics, when heat is converted into work, partially heat is rejected to cold body. For a heat engine, surrounding behaves as a cold body. Hence, it is not possible to construct an engine that will not heat into atmosphere. Q: No.24 Is it possible to convert internal energy into mechanical energy? Answer: According to Kelvin’s statement of second law of thermodynamics,” We can not construct an engine which absorbs heat from source and convert it completely into useful work without a cold body”. That is why a given amount of heat can be converted completely into work. Q: No. 25 Does entropy of the system increases or decreases due to friction? Answer: When work is done against friction, heat is produced which disturbs the molecular motion say disorder ness is created. Hence, entropy increases. Q: No. 26 An ideal reversible heat engine has 100% efficiency? Answer: No an ideal heat engine has not 100% efficiency, but it has highest efficiency than all other heat engine. Q: No. 27 Which one of the following process is irreversible? a) Slow compression of an elastic spring b) low compression of gas c) A chemical explosion d) Slow evaporation of a substance in an isolated vessel. Answer: A chemical explosion is the irreversible process. Q: No. 28 An adiabatic change in which a) Boyle’s law inapplicable b) Pressure and volume changes c) No heat is added to or taken out of a system d) No change in temperature takes place. Answer: An adiabatic change is the one in which in which, no heat is added to or taken out of a system. Q: No. 29 Give an example of a natural process that involves increase in entropy. Answer: Consider two glass of water full of water at 273 K and another glass that has water 373K.The molecules of water, in the glass are in order with their constant average velocities. Such water are mixed in a container thus disorder is created in the water molecules and will start to move from water at temperature 373K to 273 K.A engine can be operated due to this heat flow from hot water to Second YEAR GROUP TIME ------------- GROUP NO:- - - - -- - - - CHAPTER 11 HEAT 14 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #14 PTCL # 022-2670019 15 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 15 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Moving 2011 Life Towards 2012 COME FOR SUCCESS cold water and hence work will be available . This disorder increases due to mixing and difference of temperature decreases. When mixture comes to the thermal equilibrium state, disorder i.e. entropy will be maximum. Q: No. 30 . What you mean by reservoir? Answer: The thermodynamic definition of a reservoir is something large enough that it can transfer heat into or out of a system without changing temperature Q: No. 31 Why gases have two molar heat capacities? Answer: For gases, it is necessary to specify the conditions under which the change of temperature takes place, since a change of temperature will also produce large changes in pressure and volume. Thus, gas is heated at constant volume by providing fixed piston or at constant pressure by providing free piston. Hence, gases have two molar specific heat capacities. Q: No. 32 Show that, cp> cv Answer: The gas is heated by absorbing ΔQ v heat energy at constant volume. The heat energy absorbed is completely used to increase the temperature ΔT . In this system no work is to be done. Thus we know that, ΔQv = c v n ΔT The gas is heated by absorbing ΔQ p heat energy at constant pressure in a closed system. The energy supplied is partially used to increase the temperature ΔT and partially used to perform work by the system ΔW . ΔQ p = c p n ΔT This shows that, ΔQ p > ΔQ v .We know that, cp n ΔT > c v n ΔT cp n ΔT > cv n ΔT cp > c v Q: No. 33 What is Heat Engine? Answer: A device that converts heat into work is called a heat engine. The engine generally has a working substance (eg. gas or steam) that under goes thermodynamic change. The engine generally operates in a cycle which implies U = 0 (for one cycle) All heat engines can be characterized by the following: (1) They receive heat from a hightemperature source. (2)They convert part of this heat to work. (3)They reject the remaining waste heat to a low-temperature sink. (4)They operate on a cycle. Q: No. 34 What is Heat Pump Second YEAR GROUP TIME ------------- GROUP NO:- - - - -- - - - CHAPTER 11 HEAT 15 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #15 PTCL # 022-2670019 16 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 16 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Moving 2011 Life Towards 2012 COME FOR SUCCESS Answer: Heat Pump is cyclically operating device which absorbs energy form a low temperature reservoir and reject energy as heat to a high temperature reservoir when work is performed on the device. Its objective is to reject energy as heat to a high temperature body (space heating in winter). The atmosphere acts as the low temperature reservoir. T How the ratio of temperature of cold body and hot body reservoirs C affect the TH efficiency of heat engine? Answer: T T If the C ratio is greater than efficiency will be smaller or C ratio is smaller than TH TH Q: No. 35 efficiency will be greater. Because, Second YEAR T Efficiency = 1 - C is for the heat engine TH GROUP TIME ------------- GROUP NO:- - - - -- - - - CHAPTER 11 HEAT 16 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #16 PTCL # 022-2670019 17 COMPOSSED AND WRITTEN BY PROF. NAJEEB MUGHAL GOVT MUSLIM SCIENCE DEGREE COLLEGE HYD. 17 NAME - - - - - - - - - - - - - - - - - - - - S/o - D/o - - - - - - - - - - - - - - - Belongs to - - - - - - - -- - -college - - Let’s G¤ to HoUse oF sUCCess Second YEAR Moving 2011 Life Towards 2012 GROUP TIME ------------- GROUP NO:- - - - -- - - - COME FOR SUCCESS CHAPTER 11 HEAT 17 It’s Understanding, It’s Responsibility, It‘s Desire, It’s Reality, a Better shade, be share "No cyclic process is possible whose sole result is a flow of heat from a single reservoir and the performance of equivalent work." Lord Kelvin Quote Heart collection, mind satisfaction, best time for carrier, hard working, try for unlimited Education ADDRESS: Behind ALFALAH BANK, Grain Market, Branch. Prince Ali Road, adjacent G.G JAGHRANI Medicare. CELL # 0333-2602675 PAGE #17 PTCL # 022-2670019