* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ENGINEERING THERMODYNAMICS
Insulated glazing wikipedia , lookup
Dynamic insulation wikipedia , lookup
Solar water heating wikipedia , lookup
Building insulation materials wikipedia , lookup
Solar air conditioning wikipedia , lookup
Heat exchanger wikipedia , lookup
Thermoregulation wikipedia , lookup
Heat equation wikipedia , lookup
R-value (insulation) wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Cogeneration wikipedia , lookup
Intercooler wikipedia , lookup
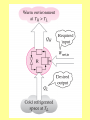
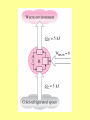
ENGINEERING THERMODYNAMICS Dr. M.R.SWAMINATHAN Assistant Professor Internal Combustion Engineering Division Department of Mechanical Engineering ANNA UNIVERSITY CHENNAI-25. LIMITATIONS OF I LAW The first law of thermodynamics is simple, general, but does not constitute a complete theory because certain processes it permits do not occur in nature! Examples of processes which are not prohibited by the first law, but cannot happen in a real world. • Perfect machine • Transfer heat from cold to hot subject • Gas expansion IRREVERSIBLE PROCESSES 1. A battery discharges through a resistor, releasing energy. The reverse process will not occur. 2. Two gases, initially in separated adjoining chambers, will mix uniformly. 3. A free expansion of gas Lussac-Joule experiment) (in Gay- 4. Heat flows from a high temperature body to a low temperature reservoir in the absence of other effect STATEMENTS OF THE SECOND LAW OF THERMODYNAMICS: Kevin-Planck Statement: It is impossible to construct a device that operates in a cycle and produces no other effects than the performance of work and the exchange of heat with a single reservoir. Model Heat Engine • Qhot= W+Qcold or • Qhot-Qcold=W (what goes in must come out) II LAW OF THERMODYNAMICS EFFICIENCY OF HEAT ENGINE Even the most efficient heat engines reject almost onehalf of the energy they receive waste heat. as It is impossible for any device that operates on a cycle to receive heat from a single reservoir and produce a net amount of work. Clausius Statement: It is impossible to construct a device that operates in a cycle and whose sole effect is to transfer heat from a cooler body to a hotter body.