* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Unit 1
X-ray photoelectron spectroscopy wikipedia , lookup
Marcus theory wikipedia , lookup
Electron scattering wikipedia , lookup
Ionic compound wikipedia , lookup
2-Norbornyl cation wikipedia , lookup
Homoaromaticity wikipedia , lookup
Heat transfer physics wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Aromaticity wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Atomic theory wikipedia , lookup
Atomic orbital wikipedia , lookup
Electron configuration wikipedia , lookup
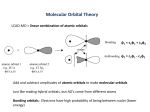
Winter ‘12 Chemistry 162 Unit 1 1/4, 1/5 Outcomes: 1. To know that chemical bonds, the forces that hold atoms together (text definition), are the lowering of energy when atoms come together (additional definition). 2. To describe, differentiate, and give examples of ionic, covalent, and metallic bonds. 3. To use Lewis dot symbols for atoms and ions as a model that shows valence electrons. 4. To use the “octet” rule to predict and describe the configuration of main group monatomic ions. 5. To know that cations are smaller than their atoms and anions are larger, and why. 6. To know that conductivity is an experimental method that can detect the presence of ions. 7. To know that ionic compounds can be formed from their elements, but most often result from combination of ions that already exist in the ionic state. 8. To know that ionic bonds result from charge attractions between individual ions throughout a crystalline lattice, without significant sharing of electrons. 9. To use Coulomb’s law qualitatively to show how charge, size, and lattice attractions affect ionic bond energies. 10. To know that the energy of ionic bonds has many components, described by the Born-Haber cycle. 11. (§8.3 content): To use “sharing of electrons” as a descriptive term for covalent bonding 12. To understand that there are orbitals that best describe the bonds, and to use energy relationships to describe the stability of covalent bonds and the energy required to break them. 13. To create and use Lewis structures to represent simple molecules. Assignments: Read §8.1, 8.2 (No Reading Quiz) Recommended exercises: From the above sections, all in-chapter Exercise and Practice problems, and select chapter-end problems referred to by the Practice problems. 1/6 (See below.) 1/9 Laboratory: Models of Molecular Structure OR Synthesis of Salicylic Acid (Preassigned on 1/4 or 1/5) Please submit the Safety Protocol signature form (p. 5 of the Lab Manual). Lab report due second class period after lab, 1/12, 1/13. 1/6, 1/10 Outcomes: 1. To use polarization (polarity) to describe covalent bonds where electron density is not symmetric and how these appear in electrostatic potential diagrams. 2. To use electronegativity to predict when bonds should be non-polar, polar, and ionic (and that there are exceptions). 3. To understand the Lewis model of electron pair bonding, and how bonding pairs and lone pairs are symbolized. 4. To know and use the rules for assigning formal charges, their limitations, and when they are useful. 5. To memorize and use Common Bonding Patterns (not in text) as a tool for predicting single bonds, multiple bonds, lone pairs, and structures. 6. To know what a resonance structure is, what is does and does not represent, and how resonance hybrids are useful for describing bonding where bonding orbitals are actually delocalized over several atoms. 7. To recognize and accept exceptions to the octet rule. 8. To recognize examples of reactions where a coordinate covalent bond forms. 9. To know and use the predictive value of bond dissociation energies and their relationship to bond lengths. 10. To use bond energies to predict enthalpies of reactions. Assignments: Read §8.3 (Reading Quiz.) Recommended exercises: From the above sections, all in-chapter Exercise and Practice problems, and select chapter-end problems referred to by the Practice problems. 1/11, 1/12 Outcomes: 1. To know that the Valence Shell Electron Pair Repulsion Model (VSEPR) uses repulsions between electron pairs to predict and describe where electron pairs and nuclei should be in polyatomic molecules and ions. 2. To describe an electron group as a set of electrons that may be in the same region of space, and to differentiate between electron group geometry and molecular geometry. 3. To use electron group geometries to describe electron arrangements around a single nucleus as linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral. 4. To use the dashed-wedge formalism to understand and communicate three-dimensional molecular structure. 5. To justify how and when molecular geometries differ from electron group geometries. 6. To use the VSEPR model to predict geometries of molecules and polyatomic ions and recognize exceptions. 7. To know that a bond dipole is a property of a bond between two atoms expressing unequal electron density. 8. To know that a dipole moment is an experimental quantity expressing the overall polarity of a molecule, reflecting the contributions of all bond dipoles and lone pairs. 9. To give examples of chemical properties and systems where polar vs. non-polar character is important. Assignments: Read §8.4, 8.5 (Reading Quiz.) Recommended exercises: From the above sections, all in-chapter Exercise and Practice problems, and select chapter-end problems referred to by the Practice problems. Lab report due at start of class for section -02. Start Graded Homework problems due 1/13, 1/17. 1/13 (See below). 1/16 Holiday (No classes or labs.) Make progress on the Chemical Resource Lab, due 1/19, 1/20. 1/13, 1/17 Outcomes: 1. To give examples of compounds that illustrate the limitations of Lewis and VSEPR models of bonding. 2. Know that bonding is best described through orbitals that are different in molecules than in atoms. 3. To know that a “basis orbital” is an atomic orbital used to form a bond, and a “bonding orbital” results. 4. To know that the Valence Bond Theory describes orbitals between two adjacent atoms while the Molecular Orbital Theory considers all atoms in a molecule. 5. To know that the concept of overlap, used in both theories, pertains to locations and energies atomic orbitals. 6. To know that the Valence Bond Theory is simpler, contains commonly used vocabulary, and works best when describing molecules without delocalization. 7. To understand that hybridization is a legitimate way of changing atomic basis orbitals before bonding, so the proper molecular geometry results. 8. To memorize and describe the geometries of sp, sp2, sp3, sp3d and sp3d2 hybrid atomic orbitals and the molecular geometries that their overlap produce. 9. To know that sigma bonds result from the end-to-end overlap of basis orbitals, and the bonding orbital includes (is wrapped around) the bond axis; to know that pi bonds result from the side-to-side overlap of basis orbitals, and do not include (existing above and below) the bond axis. 10. To know that a sigma bond is present in virtually all single bonds, and that multiple bonds are almost always comprised of one sigma bond and one or two pi bonds. 11. To understand how chemists’ communication styles refer to hybrid orbitals in molecules as if they were still present. (Finish §9.2 outcomes on 1/18, 1/19.) Assignments: Read §9.1, 9.2 (Reading Quiz.) Recommended exercises: From the above sections, all in-chapter Exercise and Practice problems, and select chapter-end problems referred to by the Practice problems. Graded Homework due at start of class: Chapter 8, #12, 24, 44, 56, 66, 80, 92 Lab report due at start of class for section -03. 1/18, 1/19 Outcomes: 1. To review examples of where a localized bonding model fails, and examples where geometries do not agree with VSEPR and Valence Bond Theories. 2. To know that in the Molecular Orbital Theory, all orbitals of all atoms in a molecule are used to determine bonding. These are usually restricted to valence orbitals, since core orbitals do not overlap. 3. To know the Molecular Orbital Theory uses atomic basis orbitals (not hybrid orbitals) and combines their wavefunctions mathematically to create new bonding and antibonding orbitals. 4. To know that bonding orbitals are lower in energy than their basis orbitals, and electrons have a high probability of being between bonded atoms. To recognize them in pictures and energy level diagrams. 5. To know that antibonding orbitals are higher in energy than their basis orbitals, and have a nodal plane between bonded atoms, and detract from bonding. To recognize them in pictures and energy level diagrams. 6. To use the output of molecular orbital calculations (energy level diagrams and probability maps) and their vocabulary to describe bonding. 7. To know the definition of bond order. 8. To use molecular orbital energy level diagrams to predict bond order, unpaired electrons, and reactivity of diatomic molecules of atoms in the first two rows of the Periodic Table. 9. To use the specific example of benzene to compare and contrast Valence Bond and Molecular Orbital descriptions of bonding where delocalization exists. Assignments: Read §9.3, 9.4 (Reading Quiz.) Recommended exercises: From the above sections, all in-chapter Exercise and Practice problems, and select chapter-end problems referred to by the Practice problems. Start Graded Homework due 1/24, 1/25 (do 66, 74 after class). Chemical Resource Lab due (section -02) 1/20 Outcomes (section -01): Start Unit 2 See Unit 2 Syllabus; reading quiz on §10.1 – 10.4. Chapter 10 content is not on the 1/25 exam. Chemical Resource Lab due (section -01) 1/23 Laboratory: Models of Molecular Structure OR Synthesis of Salicylic Acid (The experiment that was not performed previously.) Lab report due second class period after lab, 1/26, 1/27. It is strongly recommended that you complete the report before the Unit 1 hour exam. 1/24, 1/25 Outcomes: Success on an hour exam! Content from all the above learning outcomes. Graded Homework due: Chapter 9, #10, 26, 32, 34, 40, 66, 74; also Worksheet 7 Skill Development #2. Group Sheet 1