* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 9, Part 1
Homoaromaticity wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Bose–Einstein condensate wikipedia , lookup
Coupled cluster wikipedia , lookup
2-Norbornyl cation wikipedia , lookup
Cluster chemistry wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Heat transfer physics wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
State of matter wikipedia , lookup
Hartree–Fock method wikipedia , lookup
Aromaticity wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Atomic theory wikipedia , lookup
Atomic orbital wikipedia , lookup
Electron configuration wikipedia , lookup
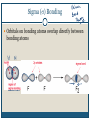
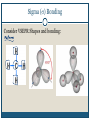
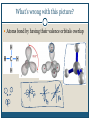
December 1, 2009 •T H E “ M A K E U P ” L E C T U R E •T O P I C S Molecular Polarity (8.7) Introduction to Bonding Theories (9.1) Valence Bond Theory (9.2) Molecular Polarity Polarity = uneven distribution of charge Bond polarity = Electrons drawn closer to the more electronegative atom Molecular polarity = Molecule as a whole has a net separation of charge A polar molecule must have polar bonds A molecule is polar if the directions of the polar bonds don’t cancel/offset eachother Nonpolar Examples: CH4, CO2 Polar Examples: H2O, NH3 Nonpolar Examples: CH4, CO2 Polar Examples: H2O, NH3 Why is Polarity Important? Polarity dictates many molecular properties Physical state (solid, liquid, gas) CO2 (44 g/mol) vs. H2O (18 g/mol) Solubility Chapter 9- Chemical Bonding Theories Valence Bond Theory: Uses Lewis Structures Bonds form using shared electrons between overlapping orbitals on adjacent atoms. Orbitals arrange around central atom to avoid each other. Two types of bonds: sigma () and pi (). Qualitative, visual- good for many atom systems in ground state Molecular Orbital Theory: Uses MO Diagrams Orbitals on atoms “mix” to make molecular orbitals, which go over 2 or more atoms. Two electrons can be in an orbital. Quantitative- needed to describe excited states Sigma () Bonding Orbitals on bonding atoms overlap directly between bonding atoms Sigma () Bonding Consider VSEPR Shapes and bonding: What’s wrong with this picture? Atoms bond by having their valence orbitals overlap Bonding orbitals are not the same shape as atomic orbitals 2pz 2px 2py 2s Orbitals in CH4 Electron configurations: H = 1s1 C = 1s22s22p2 Atomic orbitals change shape when they make molecules Hybrid Orbitals