* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 10
Survey
Document related concepts
Transcript
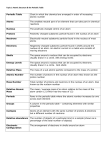
Chapter 10 1. Atom – the smallest unit of an element that maintains the properties of that element 2. Nucleus – in physical science, an atom’s central region, which is made up of protons and neutrons 3. Proton – a subatomic particle that has a positive charge and that is located in the nucleus of an atom 4. Neutron – a subatomic particle that has no charge and that is found in the nucleus of an atom 5. Electron – a subatomic particle that has a negative charge 6. Electron cloud – a region around the nucleus of an atom where electrons are likely to be found 7. Isotope – an atom that has the same number of protons but has a different number of neutrons 8. Atomic number – the number of protons in the nucleus of an atom 9. Atomic mass – the sum of the numbers of protons and neutrons in the nucleus of an atom Chapter 11 1. Periodic-describes something that occurs or repeats at regular intervals 2. Period-a horizontal row of elements in the periodic table 3. Group- a vertical column of elements in the periodic table; elements in a group share chemical properties 4. Metal-an element that is shiny and that conducts heat and electricity well 5. Nonmetal-an element that conducts heat and electricity poorly 6. Metalloid-elements that have properties of both metals and nonmetals 7. Alkali metal-one of the elements of Group 1 of the periodic table (lithium, sodium, potassium, rubidium, cesium, and francium) 8. Alkaline-earth metal-one of the elements of Group 2 of the periodic table (beryllium, magnesium, calcium, strontium, barium, and radium) 9. Halogen-one of the elements of Group 17 of the periodic table (fluorine, chlorine, bromine, iodine, and statine); halogens combine with most metals to form salts 10. Noble gas- one of the elements of Group 18 of the periodic table (helium, neon, argon, krypton, xenon, and radon); noble gases are unreactive Chapter 10 1. Atom – the smallest unit of an element that maintains the properties of that element 2. Nucleus – in physical science, an atom’s central region, which is made up of protons and neutrons 3. Proton – a subatomic particle that has a positive charge and that is located in the nucleus of an atom 4. Neutron – a subatomic particle that has no charge and that is found in the nucleus of an atom 5. Electron – a subatomic particle that has a negative charge 6. Electron cloud – a region around the nucleus of an atom where electrons are likely to be found 7. Isotope – an atom that has the same number of protons but has a different number of neutrons 8. Atomic number – the number of protons in the nucleus of an atom 9. Atomic mass – the sum of the numbers of protons and neutrons in the nucleus of an atom Chapter 11 1. Periodic-describes something that occurs or repeats at regular intervals 2. Period-a horizontal row of elements in the periodic table 3. Group- a vertical column of elements in the periodic table; elements in a group share chemical properties 4. Metal-an element that is shiny and that conducts heat and electricity well 5. Nonmetal-an element that conducts heat and electricity poorly 6. Metalloid-elements that have properties of both metals and nonmetals 7. Alkali metal-one of the elements of Group 1 of the periodic table (lithium, sodium, potassium, rubidium, cesium, and francium) 8. Alkaline-earth metal-one of the elements of Group 2 of the periodic table (beryllium, magnesium, calcium, strontium, barium, and radium) 9. Halogen-one of the elements of Group 17 of the periodic table (fluorine, chlorine, bromine, iodine, and statine); halogens combine with most metals to form salts 10. Noble gas- one of the elements of Group 18 of the periodic table (helium, neon, argon, krypton, xenon, and radon); noble gases are unreactive