* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Monoclonal antibody wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Adaptive immune system wikipedia , lookup
Innate immune system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Molecular mimicry wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
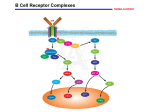
FUN2: 10:00 – 11:00 Scribe: Kallie Law Tuesday, October 28, 2008 Proof: Maggie Law Dr. Justement T and B Cell Activation Page 1 of 7 TCR – T cell receptor, TH1 – Helper 1 T cell, MHC – major histocompatibility complex, APC – antigen presenting cell, BCR – B cell receptor, smac - supra-molecular activation complex , ITAM - immunoreceptor tyrosine-based activation motifs, ITIM - immunoreceptor tyrosine based inhibitory motif B and T Cell activation Notes drawn on the board before official lecture: Cycle cell. Antigens will drive lymphocytes into the cell cycle. And when you get appropriate other signals, (these are cytokines, costimulation), then these cells will be driven through the cell cycle all the way through. So, the idea is that the first thing I want you to understand about a quiescent cell is that about enter into the cell cycle, that can be driven by antigen alone but in order for those cells to proliferate and expand, and then ultimately differentiate, you have to have other signals. You have to have costimulation, cytokines, etc. Complete lymphocyte activation requires many different signals; that is an important safety mechanism that is in place to regulate the adaptive immune response. Keep this in mind. In the process of going from G0 and entering into the cell cycle and proliferation, you have many things occur. You have changes in gene transcription, changes in the synthesis of proteins, so these are very early events that prepare the cell to enter G1 and progress to the cycle cell. The second important point is proliferation. Proliferation is important because it is what drives clonal expansion and (already mentioned) there are very few lymphocytes with any specificity in the body so you have to be able to expand their numbers in a selective way to deal with any given antigen. That is dependent on those cells have an antigen receptor that recognizes that antigen. If you think about T and B cells, the cell you start with is not the effector cell that you end up with. These cells not only go through an expansion phase, but then they must differentiate into the relevant effector cells. Again, B cells – those ultimately differentiate into plasma cells and those are the cells that are producing soluble antibody molecules. Antibodies that are secreted by the plasma cells have the same specificity as the original B cell antigen receptor. T cells – talking CD4+ --those cells, when activated, differentiate into cytokine producing cells. Differentiation is driven into many different types of helper T cells – TH1, TH2, T17 (skipped a few numbers.) These effector cells that are producing cytokines are producing cytokines that drive different aspects of the adaptive immune response. The response can be tailored to the type of pathogen and the things that need to be done to deal with that pathogen. Other major T cell is CD8+, and those differentiate into cytolytic T cells. Differentiation is very important. Critical for innate and adaptive immune response to maintain or reach homeostasis in that response. Idea is that you have to ramp up the response to deal with the initial insult. As that pathogen (bacteria, virus, etc) is dealt with, then the system has to be turned off again. Do not want to a situation where you illicit uncontrolled adaptive immune response. You want them do what they need to do and then be shut off. There is an important series of feedback mechanisms that will shut down T and B cells and return the system back to baseline. The final important point in lymphocyte activation/ adaptive immune response is memory. Memory – presumable a situation where the cells differentiate into effector cells to deal with immediate insult and at the same time, some of the cells will actually differentiate into memory cells. These cells are relatively long lived; have capacity to provide a much quicker and potent secondary response if the organism encounters the same pathogen again subsequently. These are the basic things that are critical in the lymphocyte adaptive process leading to an effective adaptive immune response. Early on gene transcription, synthesis of proteins, proliferation to promote clonal expansion of important clones that can respond to particular organism that challenges the host, differentiation into effector cells to mediate the adaptive immune response, homeostasis feedback mechanism turn on as the assault is being cleared, want to return the response to baseline. Throughout the process, you want to generate effector cells and memory cells. I. Introduction [S1]: Lymphocyte activation a. Lymphocyte activation and the basic receptors that are important in the process b. Deal with signals that are important for driving the first 2 steps. These receptors are important for the driving gene transcription, synthesis of proteins, and proliferation. c. Both T and B cells express antigen receptors. i. Antigen receptors on each T and B cell are unique in terms of their protein structure and ability to recognize an antigen. Keep this in mind. d. B cell immune receptor--membrane Immunoglobin. Has 2 heavy & 2 light chains disulfide bonded together to form the intact antigen recognition structure. e. The antigen receptor on a B cell is bivalent – 2 antigen binding sites composed of a heavy and light chain. f. Amino terminal domains or Ig domains that compose the variable heavy and light chains domains are hyper variable and confer the unique antigen recognition capability to the antigen receptor. FUN2: 10:00 – 11:00 Scribe: Kallie Law Tuesday, October 28, 2008 Proof: Maggie Law Dr. Justement T and B Cell Activation Page 2 of 7 II. Within amino domains [S2] a. Within those domains, if you stretch them out, they are about 110-120 amino acids in length. b. Analyze the amino acid utilization from many B cell receptors & you see within the variable domain of the heavy or light chain there are regions that are fairly constant. i. Regions with very little amino acids variability ii. Include hyper variable regions – 3 in each variable domains. These are called complementary determining regions. These are regions in which the amino acid usage varies greatly; confers selectively and specificity of the receptor for antigen binding. c. [S3], [S4], [S5] Concept: when you fold Ig domains in tertiary structures -–showing a globular depiction of the quaternary structure –-you see the stick lines are representing complementary determining regions. d. When you fold the variable heavy and light chain domains into their normal structure, the complementary determining domains are brought together to form the antigen binding site. e. Membrane Ig is what allows B cells to recognize antigen – called the antigen recognition structure. f. That structure is non covalently associated with a heterodimer that spans the membrane. g. BCR is non covalently associated (this structure should be shown to span the membrane and terminate with 3 amino acid tail which is positively charged to lock it into the membrane.) h. Non covalently associated with Ig alpha/Ig beta heterodime; this heterodimer is important for 2 reasons. i. Transporting the BCR to the plasma membrane ii. In the cytoplasmic domain of these peptides, there are motifs called immunoreceptor tyrosine-based activation motifs, or ITAMs. 1. Note the sequence of an ITAM – tyrosine, 2 amino acids – leucine, isoleucine, and 6-9 intervening amino acids. Then you repeat this tyrosine –XX-leucine, isoleucine motif again. 2. What happens –-when antigen binds to the BCR, you redistribute these complexes in the membrane, co localize these complexes with tyrosine kinases. Tyrosine kinases phosphorylate the tyrosine in the ITAM. 3. Create docking sites that recruit other kinases and effector proteins that initiate and propagate B cell activation. 4. Understand the core way this works. i. [SQ1]: Will we have access to this PowerPoint? i. Yes – Whikehart comes in 5 minutes later telling us that it has been uploaded to the course website. j. When you think about T cell antigen receptor – it is similar to the B cell antigen receptor but in the T cell, the antigen recognition structure, the T cell antigen receptor, has 2 polypeptides. k. Different flavors of this; most T cells in the body are alpha/beta T cells – express an alpha polypeptide which is disulfide bonded to a beta polypeptide. l. Gamma/delta T cells exist in the body; they are a smaller subset of T cells. Express a gamma and a delta polypeptide on their surface which comprises the antigen receptor. m. As with B cell antigen receptor, at the amino terminus there are 2 Ig domains- variable alpha and variable beta domain. III. Variability in the Domains [S6] a. Variability within the domains is what constitutes selectively for recognition of the antigen. b. To reiterate–-the way you encode the variability in B cell receptors and T cell receptors is that there are multiple gene elements. c. In B cell there are V genes and heavy chain elements that encode the variable domain. d. T cell receptor – alpha chain is encoded by a V and J gene element; the beta chain is encoded by a VDJ gene element. e. These are variably recombined during T and B cell development. When they are recombined in frame, these V gene elements will encode the variable domains. f. See where the CDRs are derived. In V alpha, CDR 1 and 2 is derived from the V gene element itself. CDR3 is the junction between the V and the J. g. On beta chain, see CDR 1 and 2 again encoded within the V gene element; CDR3 is encoded by the junction between V, D, and J. h. By using different V genes and recombing these genes in different outcomes, you can encode variability in the amino acids within these CDRs. IV. T cell and B cell receptors [S7] a. Note –-in the case of the T cell, the alpha and beta disulfided bonded polypeptide on the surface is what recognizes the antigen. It does not transduce a signal, but it can by virtue of being non-covalently associated to the CD3 complex and the zeta-zeta chain homodimer. b. Basic principle – these different polypeptides contain ITAMS. FUN2: 10:00 – 11:00 Scribe: Kallie Law Tuesday, October 28, 2008 Proof: Maggie Law Dr. Justement T and B Cell Activation Page 3 of 7 c. Whikehart announces that slides were just now uploaded. Sorry if it was hard to follow – I tried to work backwards to figure out what slide was being talked about 16:20. d. ITAMs are similar to what you see in the B cell receptor complex. e. You have tyrosines that get phosphorylated, and when they get phosphorylated, proteins are recruited that mediate T cell activation. f. Important distinction between T cells and B cells. g. B cells can recognize antigens derived from bacteria or viruses. h. [SQ2] It looks like it says gamma epsilon from where I am sitting. Is that a gamma epsilon T cell receptor? i. No. These are CD3. These polypeptides comprise the CD3 complex. It is a good question. There are gamma delta positive T cells. That refers to the polypeptides that comprise the antigen receptor. Understand, in addition to that (either have alpha beta or gamma delta), you also have those polypeptides being non covalently associated with the CD3 complex. CD3 complex is comprised of 2 heterodimers. One heterodimer is composed of a gamma/epsilon chain; the other heterodimer is an epsilon/delta chain. There is another gamma/delta peptide but it refers to the CD3. i. [SQ3] So the main important thing about the CD3 complex is that it can transduce signals inside the cell, whereas the alpha/beta T cell receptor cant'? i. Alpha/ beta – can’t be covalently associated. If you were to express this on the surface of a T cell with no CD3, it could bind antigen, but could not transducer the signal. It has to be non covalently associated with CD3 and this zeta zeta homodimer. These are the structures that actually transduce the signal. Same thing like the B cell receptor which is non covalently associated with the Ig alpha/beta. j. B cell antigen receptors bind to a wide range of antigens. k. B cells, via antigen receptor, recognize proteins in the form of polypeptides, RNA, DNA, lipids, carbohydrates or any combination of those. l. B cells have the ability to bind and response to a wide array of biomolecules. m. T cells are more restricted. Alpha/beta T cells – those cells, via antigen receptor, can only recognize peptides. n. The peptides are relatively short, depending on whether they are CD4 or CD8 T cells. The peptides have to be presented to the T cell by the major histocompatibility complex antigens. o. [S8] On T cells, remember they have T cell receptor complex – TCR associated with CD3 and the zeta chain homodimer, but you also have accessory molecules and you have two main types – CD4 and CD8. p. [S9]You can subset T cells based CD4 and CD8 expression and importantly, CD4 recognizes peptides that are bound to MHC Class II. CD8 recognize peptides bound MHC Class I. q. [S10] During development, T cells will ultimately express either CD4 or CD8. In conjunction with the T cell receptor that they express, this gives them the ability to recognize peptides that are presented differentially on class I or II. r. What you see on Figure 3.9 on a CD4+ T cell – that T cell via its TCR can recognize peptide presented by MHC Class II. s. CD4 is important because it can bind to non polymorphic regions in the beta 2 domain of the beta polypeptide. t. It can stabilize the interaction; important for driving a sufficient signal to promote optimal T cell activation. u. In contrast, CD8+ T cell recognize polypeptides presented by MHC Class I. CD8 plays a similar role in that it can bind to Class I and stabilize that interaction between the T cell and the APC so you get optimal activation. v. These accessory molecules are very important because they… i. increase the avidity of the interaction between the T cell and the APC. ii. Play a role in promoting signaling which can lead to T cell activation. V. Adhesion molecules [S11,12] a. There are other molecules important for T cell activation – adhesion molecules. b. See CD4 and CD8 which are the important accessory molecules that restrict T cells to MHC Class I or II. c. You also have adhesion molecules--integrins, LFA-1, LFA-2 which bind to counter ligands. i. LFA-1 binds ICAM-1 ii. LFA -3 binds to LFA-2 d. These adhesion molecules are important because they further increase stability and avidity between T cells and APC’s. e. [S13] What happens –-when T cells encounter APCs, they will first have the potential for a relatively weak interaction. f. The weak interaction is between the T cell and the APC is transient –-mediated by LFA-1, the intergrin on the T cell binding to its counter ligand which is ICAM 1 on the APC. This is a low affinity interaction. g. What this interaction does is provide enough contact between the T cell and the APC so that the TCR can scan the surface of the APC for peptides bound to MHC Class II. h. If the T cell receptor engages a peptide MHC in which it has high affinity, that will drive signaling into the T cell which alters the confirmation of the integrin, LFA-1, so now that integrin will bind with high affinity to ICAM1. FUN2: 10:00 – 11:00 Scribe: Kallie Law Tuesday, October 28, 2008 Proof: Maggie Law Dr. Justement T and B Cell Activation Page 4 of 7 i. Have binding of the TCR to the peptide MHC, binding of CD4 to MCH Class II, and high affinity binding between LFA-1 and ICAM1. j. You have multiple stabilization points between the T cell and the APC. k. [S14] Important for T cells – look at relative affinity of the TCR for binding to peptide MHC, the affinity is generally low. If you look at Antibody, i.e.. B cell Ag receptors, they show a fairly wide range of affinities. l. Wide range of affinities, but even lowest affinity Ag receptor on a B cell has higher affinity that most TCR m. Bring into play other adhesion and accessory molecules like CD4 & CD8 which will mediate the same level of affinity and avidity that you can get from the B cell when it binds Ag by itself n. Important for driving optimal T cell activation; if the interaction LFA-1 and ICAM1 is blocked, T cell activation can be blocked o. Prevent CD4 from binding to MHC Class II, T cell activation is blocked; these things cumulatively are important for T cell activation VI. Signaling [S15] a. Signaling concepts-i. T and B cell are in quiescent state; engage Ag which drives them to enter cell cycle ii. Then proliferate in response to co-stimulation and cytokines and ultimately if they receive the appropriate combination of 1. Co-stimulation 2. Cytokines then the cells will be driven to differentiate (B cells become plasma or memory cells; T cells have same basic principle.) VII. More Signaling [S16] a. This works by the following: i. On the surface of the T cell, there is a TCR in association with a CD3 complex and the zeta-zeta chain homodimer ii. In a resting T cell, these receptor complexes are distributed throughout the plasma membrane iii. When the T cell encounters and APC and the TCR engages peptide MHC on the APC, redistribution of T cell receptor complexes in the membrane iv. Receptor complex will ultimately redistribute and localize in microdomains within the membrane called lipid rafts b. This is important because the redistribution concentrates the Ag receptor complex in regions of the membrane where there are higher levels of tyrosine kinases, in particular Sarc family tyrosine kinases i. Allows for phosphorylation of the ITAMs in the cytoplasmic tail of the zeta chain or the CD3 complex. ii. Generate phosphotyrosine motifs and then important proteins that mediate further signaling can be recruited c. Slide shows schematic of redistribution process in which initially TCR complexes are distributed throughout the membrane and Ag is engaged, TCR migrate and concentrate in lipid rafts and if the locations of integrins were detected, like LFA-1 which surrounds the TCR complex or the core i. Must have TCR engaging peptide MHC which involved CD4 binding MHC and have to have integrins involved ii. Integrins form a ring around the TCR core—looks like a bull’s eye—part of immunological synapse between the T cell and the APC and is called a smac (supra-molecular activation complex) iii. TCR core is called the C-smac or the central smac iv. When you start looking at the peripheral areas with integrins and such, this is called the peripheral smac— these are things that have actually been visualized and are critical for T cell activation d. [S17] Now are going to talk about what happens downstream of this i. Once smac is formed and phosphorylation of the CD3 complex and the zeta-zeta homodimer is promoted, two steps to remember what happens next: 1. When phosphorylation is induced either a B cell or T cell receptor complex, this allows for the recruitment of additional tyrosine kinases 2. In T cell, the critical kinase is zap70—recruited and can bind to phosphorylated ITAMs because it has SH2 domains or sarcomology 2 domains (domains that can recognize phosphotyrosine) 3. This kinase can bind to the phosphorylated subunits associated with the TCR; when it is recruited and binds it gets activated and can in turn phosphorylate downstream targets 4. Downstream targets are called adaptor proteins 5. In T cells, lac and slip76 are adaptor proteins without enzymatic function, but when they are phosphorylated, they can recruit other proteins to form signaling complexes 6. Understand it at this level—don’t memorize all the proteins—the key concept it that Ag binding causes receptor redistribution that causes phosphorylation of the ITAMs in the receptor complex, a 2nd level of tyrosine kinase is recruited (in T cells, this is zap70), this kinase gets activated and FUN2: 10:00 – 11:00 Scribe: Kallie Law Tuesday, October 28, 2008 Proof: Maggie Law Dr. Justement T and B Cell Activation Page 5 of 7 phosphorylates adaptor proteins which then form the necessary complexes to mediate signaling in T cell activation. 7. Multimolecular complexes will then trigger lots of downstream signaling pathways which activate key transcription factors which promote changes in gene transcription that are required for synthesis of proteins and proliferation e. [S18] This process is true for B cells “actually I have to change this slide because each B cell receptor is only associated with one heterodimer; this is old; I really should change this—the point is that when Ag binds to the BCR, the complexes are redistributed in the membrane, they are localized in lipid rafts (have sarc family kinases which phosphorylate the ITAMs in the IGF/IG beta polypeptides)” i. When this occurs in the B cell, you recruit a tyrosine kinases called syk—homolog of zap70—in the same family ii. Syk is activated and then will phosphorylate downstream adaptor proteins iii. [S19] Blnk is a critical adaptor protein for B cells—promotes formation of a multimolecular complex which drives multiple signaling pathways which activate gene transcriptional regulators that cause changes in gene transcription process iv. Why do we care? –for example, researchers want to develop inhibitors to tyrosine kinases like syk and zap70 because if this can be done in a selective manner, lymphocyte activation can be controlled. 1. If you have an autoimmune disease and these kinases can be targeted, body would have ability to control T or B cell activation—people who are genetically deficient in zap70 have severe combined immunodeficiency 2. Understanding signaling pathways is important in identifying targets and developing drugs that can selectively inhibit the targets. VIII. Coreceptors [S20] Two More Concepts a. The first concept is in terms of B cells i. T cells only recognize Ag when it is presented to it by APC—when and where they get activated can be controlled to some extent by when and where they can engage an APC that presents Ag. ii. B cells can see Ag without it being presented to it 1. A B cell, depending on its BCR, can bind Ag in and of themselves are respond to Ag 2. Ag like polysaccharides, DNA, or RNA that are very repetitive can cause B cell activation in the absence of T cell help a. Called T-independent antigens b. Gives potential to activate B cells at any time and place; causes potential problems when B cells must be reactive against self Ag c. Coreceptors—the basic concept—have BCR and IGF/IG beta heterodimer—when that receptor complex engages Ag - it drives a signal d. Initially mediated by sarc kinases which phosphorylate the ITAMs and recruit syk which mediates further signaling e. Have coreceptors and under certain conditions, they can be brought into the process by virtue of their co-localization with their BCR—these tyrosine kinases, when activated, can phosphorylate the cytoplasmic domain of the co receptor f. One in green and one in red (co-receptors)—to denote that the coreceptors can either enhance BCR signaling and promote B cell activation or some coreceptors, when phosphorylated can attenuate the BCR signaling response and repress B cell activation 3. B cells have wide range of coreceptors—some are activating and some are inhibitory—understand that by selectively engaging different coreceptors with the Ag receptor complex under different conditions, you can either 1. Enhance or 2. Inhibit B cell activation a. Works like this: when the kinases phosphorylate the cytoplasmic domains of the coreceptors, you get motifs that are similar to ITAMs—in stimulatory coreceptors there are tyrosine bases motifs that are similar to ITAMs which recruit activated effector proteins b. Inhibitory coreceptor proteins have motifs which are called ITIMs (immunoreceptor tyrosine based inhibitory motif)—when these motifs are phosphorylated, they recruit effector proteins that can recognize phosphotyrosines (the ITIMs) that actually inhibit signaling through the BCR. b. [S21]: Examples of CD 19 and CD22 which are important coreceptors on B cells IX. Costimulation [S22] a. Basic concept—if a T or a B cell encounters Ag (some B cells can recognize Ag that don’t require T cell help— talking about T-dependent Ag for B cells in this case) and it does not get a co-stimulatory signal, then that T or B cell will be rendered innergenic FUN2: 10:00 – 11:00 Scribe: Kallie Law Tuesday, October 28, 2008 Proof: Maggie Law Dr. Justement T and B Cell Activation Page 6 of 7 b. Innergic means that it can initially respond to Ag, some signal transduction and changes in gene transcription will occur, a little bit of proliferation (cell cycle once) ---ultimately the cell will be rendered unresponsive to any subsequent encounter with the Ag. c. Cell will finally undergo apoptosis and be eliminated from the system d. If T and B cells escape the developmental process and tolerance induction and are allowed to circulate in the periphery and are specific for self antigens---if and when they encounter a self antigen---idea is that in that situation it is not likely that there will be appropriate help to provide costimulation and will only get that single Ag stimulus from the self antigen and be induced to become tolerant or innergic---cells will then undergo apoptosis and be eliminated from system. e. Normally when dealing with an infectious organism or inflammation, activation of APC occurs so they upregulate important molecules that provide costimulation—that ensures that you get an antigenic signal and costimulation—both of these are needed for productive T and B cell activation i. On the T cell, the costimulatory molecule that is important is CD28—it binds proteins in the B7 family—B7 molecules ii. 2 main types of B7 molecules, B7-1 and B7-2 which are also known as CD80 and CD86 (be aware of this terminology) iii. CD28 is constitutively expressed on T cells f. [S23] Ideally what happens is when body is exposed to an infectious organism, dentritic cells and macrophages responds to the infectious organism and the associated tissue damage and inflammation—these APC will be driven to a higher activation state, upregulate expression of B7 molecules i. When Ag is presented to T cell, the T cell will bind via TCR to the peptide MHC complex that is presented, CD4 will stabilize this, and you will get signal 1. ii. This drives the initial signal, the Ag dependent signal iii. CD28 on the T cell will engage the B7 molecules on the APC and this will drive an important 2 nd costimulatory signal that is required for optimal T cell activation g. [S24] Have to get the antigenic signal and costimulatory signal—this will drive optimal production of IL-2, upregulate of IL-2 receptors (IL-2 is very important in driving proliferation of T cells to expand the clone that is responding so you have plenty of effector cells to respond) and memory cells are also generated—this is the basic concept h. [S25] The bottom line is that if anything is done to block the CD28 signal (this slide shows a picture from the book showing multiple ways to do this), you can block T cell activation and induce apoptosis. i. Shown many ways that costimulation is critical i. [S26] Simply to say blocking C28 signaling—simple take home message—targeted as a potential therapeutic approach to deal with autoimmune disease and to deal with transplant rejection—unfortunately has not worked well yet. i. Another example of why understanding molecules and processes that are involved in T and B cell activation is important because they do represent important therapeutic targets that can have reagents developed against them. j. [S27]—skipped k. [S28] Certain Ag that certain types of B cells can responds that are called T-independent antigens—usually highly repetitive Ag and/or Ag that can not only bind to the Ag receptor but activate B cells through Toll-like receptors (innate immune cell receptors) i. T-dependent antigens are protein Ag in this case when B cells are responding to T-dependent Ag they must get costimulation 1. Signal 1 for the B cell is driven through the Ag receptor complex when it engages T dependent Ag 2. Signal on B cells is delivered by a molecule called CD40—this will bind to CD40 ligand or CD154 on activated T cells—will drive signal 2 l. [S29] In the course of a response to T dependent Ag, what happens is that APC can interact with T cells and activate them so the APC gives an antigenic signal & B7 molecule signal through CD 28 binding. i. T cell gets fully activated and then upregulates CD40 ligand so that when it encounters a B cell, the B cell can get an antigenic signal by binding Ag through its Ag receptor complex and gets 2ndary signal through CD40 when it engages CD40 ligand on the activated T cell. ii. Without the co-stimulation, B cells will not proliferate or differentiate and become inergic and they die. iii. CD40 signaling on B cells is critical for activation in driving optimal activation of T dependent Ag. m. [S30] Important—many feedback mechanisms that are involved in returning the immune response to baseline; i. Pay attention to and read up on in the book is the concept of CTLa4—expressed on T cells. ii. CTLa4 is NOT expressed on resting T cells; it is upregulated during the course of the immune response on activated T cells FUN2: 10:00 – 11:00 Scribe: Kallie Law Tuesday, October 28, 2008 Proof: Maggie Law Dr. Justement T and B Cell Activation Page 7 of 7 iii. When it gets upregulated on T cells, CTLa4 can bind to B7 molecules—important because when you drive T cell activation, you start to upregulate CTLa4—as that is expressed at higher levels, it begins to bind to B7 molecules like how CD28 did initially 1. The difference here is when CTLa4 engages B7 molecules it drives a signal that shuts the T cell down—one example of the many different feedback processes that are used to return the immune system to baseline. iv. [SQ4]: What does it shut down again? 1. CTLa4, when it is up regulated on activated T cells, binds to B7 molecules and then sends a signal to that T cell to turn that T cell off---shuts the T cell down.