* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Atomic Mass
Survey
Document related concepts
Transcript
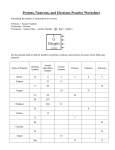
Atomic Mass Atomic Number (A) Number of protons in the nucleus Atoms of the same element all have the same number of protons In a neutral atom, the total number of protons is equal to the total number of electrons Mass Number (Z) Total number of protons and neutrons in nucleus Electrons are so small, there mass is insignificant to the atomic mass Example How many protons, neutrons, and electrons in 3H 1) 235U 2) 3) carbon-12 4) magnessium 41Ca2+ 5) 81Br 6) Isotopes Atomic masses can be different for atoms of the same element if they have different numbers of neutrons Atoms with different masses are called Isotopes or Nuclides