* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CHARACTERIZATION OF B-LACTAMASES FROM TWO B
Evolution of metal ions in biological systems wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Proteolysis wikipedia , lookup
Point mutation wikipedia , lookup
Catalytic triad wikipedia , lookup
Drug discovery wikipedia , lookup
Multilocus sequence typing wikipedia , lookup
Genetic code wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Biochemistry wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Biosynthesis wikipedia , lookup
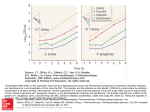
CHARACTERIZATION OF B-LACTAMASES FROM TWO BLACTAM RESISTANT ISOLATES OF PSEUDOMONAS AERUGINOSA BY E.Y. Tohamy a ; M.F. Ghaly a ; M.S. Abel-Sabourb and A.M.A.Zaida FROM a b Dept.of Botany, Faculty of Science. Zagazig University, Microbiology Dept of Genetics, Faculty of Agriculture , Moshthor, Benha branch, Zagazig University, Egypt. ABSTRACT (3-lactamase extracted from two highly (3-lactam resistant isolates of Pseudomonas aeruginosa was purified and characterized. The results of the purification steps indicate that the /3-lactamase from P. aeruginosa Z 90 was purified at 44.6 fold, the specific activity reached to 18.28 units/mgprotein and the yield percentage was reached 50.15 %, while the (3-lactamase from P.aeruginosa Z 33 was purified at 42.4 fold, the specific activity reached to 20.8 units/mg protein and the yield percentage was reached to 61.66 %. With respect to its optimum pH, the optimum values for (3-lactamase from P.aeruginosa Z 33 and P.aeruginosa Z 90 were 7.5 and 8.0, respectively. The optimum temperature was around 40°C for both enzymes from the two isolates. Studying the heat stability of (3-lactamase revealed that the enzyme of P.aeruginosa Z90 was more stable than the enzyme from P.aeruginosa Z 33. The enzyme activity was increased linearly with increasing (3-lactamase concentration with small deviation of linearity at higher concentrations in the two isolates. Amino acid analysis of the purified (3-lactamase from the two isolates indicate that alanine was the most abundant amino acid in the two isolates. Isolucine was the least detected amino acid in (3-lactamase from P.aeruginosa Z 90 with a percent of 3.12 % while tyrosine was the least one in P. aeruginosa Z 33 with a per cent of 1.93 %. The results of inhibition profiles show that (3-lactamase from the two isolates was strongly inhibited by 0.1 mM CuSO^ but not by EDTA. (3-lactam inhibitors results indicated that carbenicillin was the stronger inhibitor for the purified (3-lactamase from P.aeruginosa Z 33, while amoxycillin was the stronger one for the enzyme from P.aeruginosa Z 90. INTRODUCTION p-lactamase has been considered one of the biochemical mechanisms of resistance to p-lactam antibiotics in bacteria (Sotto et al., 1996). The enzymes from Grampositive bacteria are inducible, extra cellular and have predominantly penicillinase activity but the enzymes from Gram- negative bacteria are more varied in nature, but may be classified into several groups by substrate profile, inhibitory profile and physicochemical and immunological properties (Sato et al., 1982 and Bush et al., 1991). Az. J. Microbiol Vol. 53, July, 2001. Pseudomonas aeruginosa is, an opportunistic human pathogen, responsible for several infections mainly in immunocompromised hosts (Grimwood, 1992; Bert and Lambert-Zechovsky, 1996 and Hostacka & Majtan, 1997). These bacteria cause several infections which are associated with urinary, respiratory, burn wounds, eyes, ear, as well as nosocomial infections (Ashour, 1980; Kadry, 1985 Hoiby et a/.,1987; Tummler et al., 1991 and Ravaoarinoro et a!., 1996). The emergence of resistance to the antibiotics specially aminoglycoside and |3lactam antibiotics is a major problem in treating infections with P.aeruginosa (Radberg et a/., 1990). Bacterial resistance to P-lactam antibiotics is often associated with the production of p-lactamases which hydrolyze the cyclic amide bond in the (3-lactam ring of penicillins, cephalosporins and related compounds (Abraham and Chain, 1940; Bush et al., 1991; Mascaretti et al., 1999 and Yan et al., 2000). This study aimed to purify and characterize the properties of Plactamase from two highly resistant isolates of clinical P.aeruginosa. MA TERIALS AND METHODS Tested P.aeruginosa isolates: , Two P-lactam resistant isolates of Pseudomonas aeruginosa were isolated from documented infections at Zagazig University Hospitals, Egypt as described previously (Ghaly et al., 2001). These isolates were identified by both the traditional diagnostic biochemical tests according to Wong et al., 1984; Degtyar et al., 1985 and Gilardi, 1971 and 1992 as well as with the API 2oE system. These two isolates were designated as P.aeruginosa Z 33 and P.aeruginosa Z 90. The two tested P.aeruginosa isolates were described for their antibiotics susceptibility by the standard agar disc diffusion test according to Bauer et al. (1966) using Mueller-Hinton agar plates (Difco). The results were interpreted according to the National Committee for Clinical Laboratory Standards (NCCLS, 1999), guidelines were followed wherever possible. P.aeruginosa Z 33 and Z 90 tested in the present study were multiple resistant to at least 8 antibiotics namely; ampicillin, amoxicillin, amoxycillin/clavulanic acid (2:1), cephalexin. cephaloridine, cloxacillin, ceftraiaxone, and cefotaxime (Ghaly et al., 2001). Preparation of p-lactamase extract (as described by Pagani et al., 1990): Two highly P-lactam resistant isolates of P. aeruginosa were grown overnight in brain heart infusion broth (Difco), then diluted 10-fold with the same medium and cultured with shaking at 37 C for 5 h. After 5h of incubation, the bacterial cells were harvested by centrifugation at 4 C, washed twice with 50mM phosphate buffer [pH: 7.0] and suspended in 4 ml of the same buffer. The cell suspension was sonicated in ultrasonic disrupter (UR-150 P; Tomy Seiko Co., Ltd) at 75 W for 3 min. in an ice bath. The disrupted cell suspension was centrifuged at 10000 ipm for 15 min. at 4 C, the resulting supernatant was used as a crude enzyme extract. 110 Az.J. Microbiol. Vol. 53, July, 2001. Purification of p-lactamase (according to Cuchural et a/., 1986): The crude enzyme solution was subjected to ammonium sulphate precipitation (50-90%). The solutions were precipitated by centrifugation at 10000 rpm, then each fraction precipitate was dissolved in 5 ml of 0.1 M phosphate buffer [pH: 7.0]. p-lactamase activity was measured and the protein concentration were determined in each fraction according to Lowrey et al., 1951 with bovine serum albumin as standard.The active protein fractions were applied to a sephadex G-100 (Sigma Co.) gel filtration column equilibrated with 0.01M potassium phosphate buffer [pH 8.0] and eluted with the same buffer . Active fractions were applied to a DEAE -DE 52 -cellulose anion exchange column equilibrated with 0.01 M potassium phosphate buffer [pH 8.0] and eluted with 0.05 M NaCl in 0.01M phosphate buffer. All purification steps were performed at 4°C. P -lactamase assay: p-Lactamase activity of the crude enzyme and the active fractions in different steps was assayed spectrophotometrically with nitrocefin according to O' Callaghan et a/.(1972) and Neu (1989). One unit of p-lactamase activity was defined as that amount of enzyme that can destroy 1 (i mol of nitrocefin per min. P-lactamase specific activity of a sample was calculated by dividing the Plactamase activity by the amount of protein (units per milligram) in the reaction mixture. B- lactamase activities from the two tested P.aeruginosa isolates were tested for their optimum pH, optimum temperature, heat stability, substrate concentration and p-lactamase concentration, according to O,Callaghan et al., 1972 and Cuchural et al., 1986. Amino acid analysis: Amino acid analysis was performed on reverse phase-high pressure liquid chromatography (Shimadzu LC-10AD, Shimadzu Corporation, Japan) following hydrolysis of the sample with 6N HC1 at 110°C for 24 h according to (Association of Official Analytical Chemists AOAC (1995). Samples were analyzed on Shimpack amino-Na type column (10 x 6 mm) obtained from Shimadzu Corporation. The post column samples were derivated with O-phthaldialdehyde (OPA) according to Ishida et al. (1981) and data were integrated using an integrator model CR7A (Shimadzu chromatopac data processor). Influences of some non (Mactam and p-Iactam compound on B-Iactamases activities: The purified p lactamases from the two isolates of P.aeruginosa (Z 33 and Z 90) were preincubated with the following compounds for 5 min before the addition of nitrocefin, the non B-lactam compounds were the following: EDTA and iodine with a concentration of (lOmM), Cuso4, CoSO4, andFeS04 with a concentration of 0.1 mMand MgSO4, mNso4 and CaSO4 with a concentration of IMm. The PIactam compounds used were the following p-lactam antibiotics: penicillin, carbenicillin, cefotaxim, oxacillin and amoxycillin with a concentration of (0.1 Mm). After the incubation period p-lactamase activities were measured. Al. J. MicrobioL Vol. 53, July, 2001. RESULTS AND DISCUSSION The susceptibility of the two studied isolates of P. aeruginosa against p-lactam antibiotics were summarized in Table 1. The data revealed that P. aeruginosa Z 90 was resistant to the action of all tested antibiotics with the exception of imipenem, whereas P. aeruginosa Z 33 was resistant to the action of all tested antibiotics with the exception of imipenem and ceftazidime. These results were in agreement with that of Williams (1985), where he reported that imipenem has a high potency against a broad spectrum of microorganisms including P. aeruginosa. Production of P-lactamase is a major factor in the emergence of resistance during antimicrobial therapy and associated relapses of infection (Letendre and Turgeon, 1989). Production of P-lactamase from bacteria was reported by many investigators (Cuchural et al., 1986; Fujii et a/., 1986 Edwards & Green wood, 1990 and Sotto et al., 1996). The results showed in Fig. 1 (a,b) revealed that 80% saturation of ammonium sulphate gave maximal value of protein and p-lactamase activity in the two studied P.aeruginosa isolates (Z 33 and Z 90), where the maximum P-lactamase activities were 5.8 and 2.9 u mol/min/ml for the two isolates, respectively. This result was in agreement with the result of Cruchural et al., 1986. The results of the purification steps (Table 2, Fig 2(a.b) and Fig. 3(a,b) indicate that P-lactamase from P.aeruginosa Z 90 was purified at 44.6 fold and the specific activity reached to 18.28 units/mg protein and the yield percentage was reached to 50.15 % while the p-lactamase from P. aeruginosa Z 33 was purified at 42.4 fold and the specific activity was reached to 20.8 and the yield percentage was reached to 61.66%. The extremely low specific activities of enzymes from more resistant isolates may remain a problem ( Britz and Wilkinson, 1978). In the present investigation , the results (Fig.4) revealed that the optimal pH of Plactamase varied with the strains. The values were 7.5 and 8.0 for P-lactamase from P.aeruginosa Z 90 and P.aeruginosa Z 33, respectively. This result nearly coincided with the result of Furth, 1975, where he reported that pH:8 was the optimum for P-lactamase from P. aeruginosa strain Dalgleish. The small diversity of optimum pH values may be related to strain of P. aeruginosa used or more likely to the substrate and/or the buffer used in detecting the activity. The effect of temperature on p-lactamase activity is shown in Fig.5. The results show that the optimum temperature was around 40°C for the two P.aeruginosa isolates. In this experiment, P-lactamase activity was assayed at the optimum pH for each isolates. The optimum temperature recorded herein is quite similar to that recorded by Saino et al. (1982). An experiment was designed to study the heat stability of the purified Plactamase from the two studied P. aeruginosa isolates at 60 C. The results (Fig.6) show that P-lactamase of P. aeruginosa Z 90 was more stable than that of P.aeruginosa Z 33. Studying the effect of enzyme concentration on p-lactamase activity (Fig.7), resulted in a linear relationship but the straight line deviated from linearity at the higher concentrations. Also the p-lactamase activities from the two highly resistant isolates of P. aeruginosa were measured at different concentrations of nitrocefm, starts from 5ul (2.5 ug) up to 50 ul (25 ug). The results obtained 112 Az. J. Microbiol. Vol. 53, July, 2001. (Fig.8) show that, at lower concentrations, the rate of P-lactamase reaction resulted in a curve with a sort of linearity. As the concentration of substrate increased, this linearity decreased until any further increase in the substrate concentration did not show significant increase or expressed a little decrease in the velocity of the enzymatic reaction. Indeed, the present results are in accordance with the typical enzymatic reaction given earlier by Michaelis and Menten(1913). Amino acid composition of the purified P-lactamase was determined from the two studied P.aeruginosa isolates. The results shown in Table 3 revealed that fourteen amino acids were detected with alanine being the most abundant amino acid in the two isolates, similar results were reported by Kesado etal. (1989). Isolucine was the least detected amino acid in P.aeruginosa Z 90 with a percent of 3.12 % whereas tyrosine was the least one in P.aeruginosa Z 33 with a percent of 1.93%. For evaluating the role of metal ions and chelating agent (EDTA) on the Plactamase activity, eight compounds were studied namely; EDTA, iodine (10 mM), CuSO4, CoSO4, FeSO4 (0.1 mM) and Mg SO4, Mn SO4, Ca SO4 (ImM). The purified p-lactamase from the two studied isolates was pre incubated with a test compound in 50 mM phosphate buffer at the optimum temperature and pH values for each isolates. After the incubation period (20 min.), the remaining Plactamase activity was assayed spectrophotometrically at 482 nm with nitrocefin as substrate. The data present in Table 4 revealed that the enzyme from the two studied isolates (Z90 and Z 33) was inhibited by CuSO4, CoSO4 and iodine but not inhibited by EDTA. The results of this investigation were in accordance with that of Saino et al. (1982) where they reported that the P-lactamase activities from Pseudomonas maltophlia were inhibited by iodine and CuSO4. On the other hand, the inhibitory effect of 10 mM Cor+ which observed in this investigation was in disagreement with the result of Fujii et al. (1986) on the P-lactamase from Legionella gormanii. Generally, the inhibition profiles of P-lactamase differs with the source of the enzyme. Fe^+ caused activation for the P-lactamase extracted from P. aeruginosa Z33 more than P.aeruginosa Z 90. Mg^+, Mn^+ and Ca^+ stimulated the P-lactamase activities from the two P.aeruginosa isolates with the exception of Mg2+ which not stimulate the enzyme from P.aeruginosa Z 90. Britz and Wilkinson (1978) reported that the addition of ImM CaC^, MnC^ or MgSO4 did not alter the activity of P-lactamase from Bacteroides fragilis. The effect of p-lactam compounds on the purified p-lactamase from the two P.aeruginosa isolates (Z33, Z90) are shown in Table 5. Five p-lactam inhibitors with concentration of O.lmM were used namely; penicillin G, carbenicillin, cefotaxim, oxacillin and amoxycillin. The results are expressed as percentage of control. The inhibitors could be arranged regarding to their effect on the enzyme in the following sequence as carbenicillin > penicillin G > amoxycillin > oxacillin > cefotaxim for the enzyme from P. aeruginosa Z33. On the other hand, they were as; amoxycillin > oxacillin > carbenicillin > cefotaxim > penicillin G for the enzyme from P. aeruginosa Z90, these results confirm the results of Britz and wilkinson (1978). Az. J. Microbiol. Vol. 53, July, 2001. 113 Table (1): Antibiotic susceptibility of the two clinical P.aeruginosa isolates (Z 33 and Z 90). Antibiotic* Potency P.aeruginosa Z33 P. aeruginosa Z 90 Ampicillin 10 ug R R Amoxycillin 20 ug R R Amoxycillin/clavulanic acid (2:1) 30 ug R R Cephalexin 30 ug R R Cephaloridine 30 ug R R Cloxacilin 5ug R R Ceftriaxone 30 ug R R Ceftazidime 30 ug S R Cefotaxim 30 ug R R Imipenem 10 ug s s *AI1 antibiotic discs were purchased from Oxoid Co., England except methicillin which was purchased from BioMerieux Co.,France. R : Resistant S : Susceptible Table (2): Purification steps of p-Iactamases from P. aeruginosa Z 90 and P. aeruginosa Z 33. Activity Protein Specific Purification yield % Purification steps (umol/min/ml) (mg/ml) activity (units/nig) fold 6.56 14.69 0.41 1 100 Ammonium sulphate 5.63 10.57 0.53 1.29 85.82 SephadexGlOO 4.12 3.63 1.13 2.76 62.80 DEAE Cellulose 3.29 0.180 18.28 44.59 50.15 Crude extract 5.06 10.30 0.49 1 100 Ammonium sulphate 4.35 7.50 0.58 1.18 85.97 SephadexGlOO 4.09 2.43 1.68 3.43 80.83 DEAE Cellulose 3.12 0.15 20.8 42.4 61.66 aeruginosa Z 90 Crude extract icruginosa Z 33 Az. J. Microbiol. Vol. 53, July, 2001. Table (3): Amino acid composition of p-lactamase (mixed fractions) from two p-lactam resistant isolates of P.aeruginosa (Z 90 and Z 33). Amino acid The total detected amino acids ( % ) P.aeruginosa Z 90 P.aeruginosa Z 33 Aspartic acid 10.38 11.10 Threonine 5.40 6.20 Serine 5.87 6.73 Glutamic acid 11.73 12.52 Glycine 9.93 11.48 Alanine 13.09 14.44 Valine 4.84 6.15 Isoleucine 3.12 3.45 Leucine 9.26 8.42 Tyrosine 3.66 1.93 Phenylalanine 3.64 2.66 Histidine 6.87 2.94 Lysine 6.26 6.39 Arginine 5.95 5.59 Table (4): Influences of non- p-lactam compounds on the activity of Plactamase from P.aeruginosa Z 90 and Z 33. Non p-lactam compound P-lactamase activity as % of control P.aeruginosa Z 90 P.aeruginosa Z 33 EDTA(lOmM) 101.19 101.02 Iodine (lOmM) 96.40 94.81 CuSO4(0.1mM) 58.86 60.63 CoSO4(0.1mM) 92.19 92.72 FeS04(0.1mM) 104.20 129.52 M 92.67 124.66 198.92 316.89 § S04 (ImM) Mn S04 (lmM) Ca SO4 (imM) 256.81 352.06 Table (5): Influences of p-lactam compounds on the activity of the purified Plactamase from P.aeruginosa Z 90 and Z 33. p-lactam compound (O.lmM) P-lactamase activity (as % of control) P. aeruginosa Z 90 Penicillin G Carbenicillin 95.665 28.259 36.684 Cefotaxim Oxacillin Amoxycillin 27.452 18.479 P. aeruginosa Z 33 35.165 32.454 43.829 39.739 38.771 Az. J. Microbiol. VoL 53, July, 2001. 121 REFERENCES Abraham, E.P. and Chain, E.(l940): An enzyme from bacteria able to destroy penicillin. Nature., 146: 837. Ashour, M.S.M. (1980): Studies on the therapy of burns in experimental animals subjected to infection with Pseudomonas aeruginosa isolated from cases in Egyptian hospitals. Ph.D. Thesis, College of Pharmacy, Cairo Univ. Association of official analytical chemists (AOAC). (1995): Official methods of analysis .16 th edn. Association of official analytical chemists, Washington, DC. Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C. and Turck, M.(1966): Antibiotic susceptibility testing by a standard single disc method. Am. J. Clin. Pathol., 45: 493-496. Bert, F. and Lambert-Zechovsky, N. (1996): Comparative distribution of resistance patterns and serotypes in Pseudomonas aeruginosa isolates from intensive care units and other wards. J. Antimicrob. Chemother.,37: 809-813. Britz, M.L and Wilkinson, R.G. (1978): Purification and properties of betalactamase from Bacteroidesfragilis. Antimicob. Agents Chemother.,13: 373-382. Bush, K.; Flamm, R.K.; Ohringer, S.; Singer, S.B.; Summerill, R.B. and Banner, D.P. (1991): Effect of clavulanic acid on activity of p-lactam antibiotics in Serratia marcescens isolates producing both a TEM P-lactamase and a chromosomal cephalosporinase. Antimicrob. Agents Chemother., 35: 2203-2208. Cuchural, G.J.; J.R.; Malomy, M.H. and Tally, P.P. (1986): p-lactamase mediated imipenem resistance in Bacteroids fragilis. Antimicrob. Agents Chemother., 30: 645-648. Degtyar, N.V.; Litovchendo, P.P.; Znamensky, N.A.; Abu El-Hawa, M.W.; Nazaarchulk, L.V. and Maximets^i.P. (1985): Identification of Pseudomonas aeruginosa and similar Gram-negative glucose non-fermenting bacteria using a minimal number of tests. Lab.Delo., 5: 311. Edwards, R. and Greenwood, D. (1990): Effect of growth conditions and storage on the specific activity of J3-lactamases of Bacteroids spp. J. Appl. Bacteriol., 69:421-425. Fujii, T.; Sato, K.; Miyata, K.; Inoue, M. and Mitsuhashi, S. (1986): Biochemical properties of p-lactamase produced by Legionella gormanii. Antimicrob. Agents Chemother., 29: 925-926. Furth, A.G. (1975): Purification and properties of a constitutive P-lactamase from Pseudomonas aeruginosa strain Dalgleish. Biochemica et Biophysica Acta., 377 : 431-443. 122 Az. J. Microbiol. Vol. 53, July, 2001. Ghaly, M.F.; Tohamy^ E. Y.; Abdel-Sabour, S.; Kadry, A.A.; ABOU-EL-Hawa, M. and Zaid, A..M.A. (2001): Resistance patterns, Plasmid profiles and Blactamases of Clinical isolates of Pseudomonas aeruginosa. Under puplication. Grimwood, K. (1992): The pathogenesis of Pseudomonas aeruginosa lung infections in cystic fibrosis. J. Pediat. Child. Health .,28:4 Gilardi, G.L. (1971): Characterization of Pseudomonas aeruginosa species isolated from clinical specimens . Appl. Microbiol., 21: 414-419. Gilardi, G.L. (1992): Pseudomonas and related genera . In: Manual of Clinical Microbiology, 5 th ed. A. Balows ; W.J. Hausler ; K.L. Hermann ; H.D. Isenberg and H.j. Shadomy , Washington , D.C. American Society for Microbiology., 5: 2763-2776. Hoiby, N.; Doring, G. and Schiotz, P.O. (1987): Pathogenic mechanisms of chronic Pseudomonas aeruginosa infections in cystic fibrosis patients . Antibiot. Chemother., 39:60Hostacka, A. and Majtan, V. (1997): Serotyping and virulence factors of Pseudomonas aeruginosa clinical isolates. Acta Microbiologica et Immunologica Hungarica .,44: 141-146. Ishlda, Y.; Fujita, T.; andAsai, K. (1981): New detection and separation method for amino acids by high-performance liquid chromatography. J. Chemother., 204: 143-148. Kadry, A.A. (1985): Antibiotic inactivating enzymes of certain strains of P.aeruginosa. Faculty of Pharmacy, Zagazig Univ. Kesado, T.; Lindqvist, L.; Hedberg, M.; Tuner, K. and Nord ,C.E. (1989): Purification and characterization of a new (3-lactamase from Clostridium butyricum. Antimicrob. Agents Chemother., 33: 1302-1307. Letendre, E.D. and Turgeon, P.L. (1989): Production and induction of (3lactamase during growth of Pseudomonas aeruginosa in biological fluids. Antimicrob. Agents Chemother., 33: 776-777'. Lowry, O.H.; Rosebrough, N.J.; Farr, A.L. and Randall, R.J. (1951): Protein measurement with the folin phenol reagent. J. Biol. Chem., 193: 265275. Mascaretti ,O.A.; Danelon, G.O.; Laborde, M.; Mata, E.G. and Setti, E.L. (1999): Recent advances in the chemistry of beta-lactam compounds as selected active site serine beta-lactamase inhibitors. Curr. Pharm. Des.,5: 939-953. Michaelis, L. and Menten, M.L. (1913): Die kinetik der invertin wirking. Biochem. Z., 49: 333-369. National Committee for Clinical Laboratory Standards (NCCLS). (1999): Performance standard for antimicrobial susceptibility testing. Document M2-A6 and M7-A4. January 1999. Pennsylvania, USA. Az, J. Microbiol. Vol 53, July, 2001. 123 Neu, H. (1989): In vitro activity of P-lactamase stability of a new carbapenem, SM-7338. Antimicob. Agents Chemother., 33: 1009- 1018. VCallaghan, H.C.; Morris, A.; Kirby, S.M. andShingler, A.H. (1972): Novel method for detection of p-lactamases by using a chromogenic cephalosporins substrate. Antimicrob. Agents Chemother., 1: 283 - 288. Pagani, L.; Landini, P.; Luzzaro, F.; Debiaggi, M. andRomero, E. (1990): Emergence of cross-resistance to imipenem and other p-lactam antibiotics in Pseudomonas aeruginosa during therapy. Microbiologica., 13: 43-53. Radberg, G.; Nilssen, L.E. and Svensson, S. (1990): Development of quinolone imipenem cross resistance in Pseudomonas aeruginosa during exposure to ciprofloxacin. Antimicrob. Agents Chemother., 34: 2142-2147. Ravaoarinora, M.; Mohapatra, S.; Shore, J.; Rawal, S.; Omri,A.; Yaghi, J. and Oriol, F. (1996): Serotyping clinical isolates of Pseudomonas aeruginosa in relation to infection site , antibiotic susceptibility and beta-lactamase production. Inter. J. Antimicrob. Agents., 7: 65-68. Saino, ¥.; Kobayashi, F.; Inoue, M. and Mitsuhashi, S. (1982): Purification and properties of inducible penicillins P-lactamase isolated from Pseudomonas maltophilia. Antimicrob. Agents Chemother., 22: 564-570. Sato, K.; Matsuura, Y.; Inoue, M. and Mitsuhashi, S. (1982).'Properties of a new penicillinase type produced by Bacteroides fragilis. Antimicrob. Agents Chemother., 22:579-584. Sotto, A.; Brunchwig, C.; O,Callaghan, D.; Ramuz, M. and Jourdan, J. (1996): In Vitro evaluation of P-lactamase inhibition by latamoxef and imipenem. J. Antimicrob. Chemother., 37 :697-701. Tummler, B.; Koopmann, U.; Grotues. D.; Weissbrodt, H.; Steinkamp, G. Von der Hardt , H. (1991): Nosocomial acquistion of Pseudomonas aeruginosa by cystic fibrosis patients.J.Clin. Microbiol., 29: 1265-1267. Williams, J.R. (1985): Activity of imipenem against Pseudomonas and Bacteriodes species. Rev. Infect. Dis., 7: 411-416. Wong, K.; Roberts, M.C.; Owens, L.; File, M. and Smith, A.L. (1984): Selective media for the quantitation of bacteria in cystic fibrosis sputum. J. Med. Microbiol., 17: 113-119. Yan, J.J.; Wu, S.M.; Tsai, S.H.; Wu, J.J. and Su, LJ. (2000): Prevalence of SHV-12 among clinical isolates of Klebsiella pneumoniae : identification of a novel Ampc enzyme ( CMY-8) in southern Taiwan. Antimicrob. Agents Chemother., 44: 1438-1442.