Chapter 4-Arrangement of Electrons in Atoms
... Section 6.2: Quantized Energy- Photons 1. Max Planck: Define quantum. 2. Using the equation below, calculate the following. E= h x v ...
... Section 6.2: Quantized Energy- Photons 1. Max Planck: Define quantum. 2. Using the equation below, calculate the following. E= h x v ...
Chapter 5 Sec. 2 Bohr`s Model and the Quantum Mechanical Model
... o In 1924, a French physics student named Louis de Broglie explained the fixed energy levels of Bohr’s model. He explained that electrons can act like _____________________________. He also showed that electrons on circular orbits can only have _____________________ numbers of wavelengths. o de ...
... o In 1924, a French physics student named Louis de Broglie explained the fixed energy levels of Bohr’s model. He explained that electrons can act like _____________________________. He also showed that electrons on circular orbits can only have _____________________ numbers of wavelengths. o de ...
spectral lines
... Bohr to the rescue In 1905, Einstein had proposed the wave/particle duality of light with the photon. In 1913, Bohr used the concept in creating a quantum model of the atom. ...
... Bohr to the rescue In 1905, Einstein had proposed the wave/particle duality of light with the photon. In 1913, Bohr used the concept in creating a quantum model of the atom. ...
CHAPTER 4 TEST REVIEW GUIDE
... Students should be able to: 1. Explain why electromagnetic radiation is thought of as having dual nature. ...
... Students should be able to: 1. Explain why electromagnetic radiation is thought of as having dual nature. ...
De Broglie and Heisenberg
... Heisenberg's uncertainty principle tells us that it is impossible to simultaneously measure the position and momentum of a particle with infinite precision. In our everyday lives we virtually never come up against this limit, hence why it seems peculiar. In a modern single slit experiment, a laser i ...
... Heisenberg's uncertainty principle tells us that it is impossible to simultaneously measure the position and momentum of a particle with infinite precision. In our everyday lives we virtually never come up against this limit, hence why it seems peculiar. In a modern single slit experiment, a laser i ...
The Particulate Nature of Light
... - e.g., the deflection of a stream of electrons is just like the path of a projectile. Electrons can also exhibit wave properties - e.g., they can demonstrate diffraction. De Broglie proposed that an electron of mass m moving with speed v would have a wavelength given by: = h/p This equation gives ...
... - e.g., the deflection of a stream of electrons is just like the path of a projectile. Electrons can also exhibit wave properties - e.g., they can demonstrate diffraction. De Broglie proposed that an electron of mass m moving with speed v would have a wavelength given by: = h/p This equation gives ...
Physics 120 Homework Set #1 (due Sunday
... with each quanta is very large and only a few oscillators will have this much energy at any finite temperature. 2) a) What is particle-wave duality? b) Explain how Einstein’s equations for the energy and momentum of light quanta were used by De Broglie to encapsulate this duality. c) Identify the pa ...
... with each quanta is very large and only a few oscillators will have this much energy at any finite temperature. 2) a) What is particle-wave duality? b) Explain how Einstein’s equations for the energy and momentum of light quanta were used by De Broglie to encapsulate this duality. c) Identify the pa ...
3quarksdaily: More Is Different
... quantum mechanics. At the turn of the last century, the reluctant revolutionary Max Planck was forced to declare a resolution to a set of problems that had plagued physicists for years. All these contradictions would disappear, he grudgingly said, if one assumed that energy could only be radiated an ...
... quantum mechanics. At the turn of the last century, the reluctant revolutionary Max Planck was forced to declare a resolution to a set of problems that had plagued physicists for years. All these contradictions would disappear, he grudgingly said, if one assumed that energy could only be radiated an ...
photon particle - wave duality
... 1. a. Read sections 2.1 and 2.2 of the text. What is the ultraviolet catastrophe of the Rayleigh-Jeans law (equation 2.5)? b. Read section 2.3 paying particular attention to assumption (1) and (2) on page 47. What does the Maxwell - Boltzmann distribution function (equation 2.6) tell you about the n ...
... 1. a. Read sections 2.1 and 2.2 of the text. What is the ultraviolet catastrophe of the Rayleigh-Jeans law (equation 2.5)? b. Read section 2.3 paying particular attention to assumption (1) and (2) on page 47. What does the Maxwell - Boltzmann distribution function (equation 2.6) tell you about the n ...
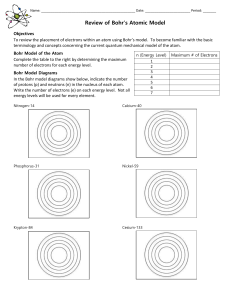
Name: ______ Date: Period: ______ Review of Bohr`s Atomic Model
... Review of Bohr’s Atomic Model Objectives ...
... Review of Bohr’s Atomic Model Objectives ...
Quantum mechanics
... Quantum mechanics – The new way that was developed at the beginning of the 20th century to interpret & predict behaviors of microscopic objects such as atoms, electrons, .. ...
... Quantum mechanics – The new way that was developed at the beginning of the 20th century to interpret & predict behaviors of microscopic objects such as atoms, electrons, .. ...
Lecture 1
... oscillated between a mechanical and an undulatory conception of light; however, these two views are perhaps less opposed to one another than was previously thought, and the development of quantum theory, in particular, appears to confirm this view. On the basis of the idea of a generally valid relat ...
... oscillated between a mechanical and an undulatory conception of light; however, these two views are perhaps less opposed to one another than was previously thought, and the development of quantum theory, in particular, appears to confirm this view. On the basis of the idea of a generally valid relat ...
Bohr–Einstein debates

The Bohr–Einstein debates were a series of public disputes about quantum mechanics between Albert Einstein and Niels Bohr. Their debates are remembered because of their importance to the philosophy of science. An account of the debates was written by Bohr in an article titled ""Discussions with Einsteinon Epistemological Problems in Atomic Physics"". Despite their differences of opinion regarding quantum mechanics, Bohr and Einstein had a mutual admiration that was to last the rest of their lives.The debates represent one of the highest points of scientific research in the first half of the twentieth century because it called attention to an element of quantum theory, quantum non-locality, which is absolutely central to our modern understanding of the physical world. The consensus view of professional physicists has been that Bohr proved victorious, and definitively established the fundamental probabilistic character of quantum measurement.