Chapter28ReadingQuiz..
... electrons have a wave nature. light has a particle nature. a photon can be converted into an electron. electrons are the conductors in metals. ...
... electrons have a wave nature. light has a particle nature. a photon can be converted into an electron. electrons are the conductors in metals. ...
On the Einstein-Podolsky-Rosen paradox
... With the example advocated by Bohm and Aharonov [6], the EPR argument is the following. Consider a pair of spin one-half particles formed somehow in the singlet spin state and moving freely in opposite directions. Measurements can be made, say by Stern-Gerlach magnets, on selected components of the ...
... With the example advocated by Bohm and Aharonov [6], the EPR argument is the following. Consider a pair of spin one-half particles formed somehow in the singlet spin state and moving freely in opposite directions. Measurements can be made, say by Stern-Gerlach magnets, on selected components of the ...
WAVE MECHANICS (Schrödinger, 1926)
... * The energy depends only on the principal quantum number, as in the Bohr model: En = -2.179 X 10-18J /n2 * The orbitals are named by giving the n value followed by a letter symbol for l: l= 0,1, 2, 3, 4, 5, ... s p d f g h ... * All orbitals with the same n are called a “shell”. All orbitals with t ...
... * The energy depends only on the principal quantum number, as in the Bohr model: En = -2.179 X 10-18J /n2 * The orbitals are named by giving the n value followed by a letter symbol for l: l= 0,1, 2, 3, 4, 5, ... s p d f g h ... * All orbitals with the same n are called a “shell”. All orbitals with t ...
Synopsis
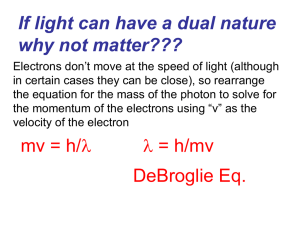
... Don’t try to work out the momentum of a high speed particle and hence its de-Broglie wavelength using its rest mass. Mas increase significantly as the speed of the particle approaches the speed of light! ...
... Don’t try to work out the momentum of a high speed particle and hence its de-Broglie wavelength using its rest mass. Mas increase significantly as the speed of the particle approaches the speed of light! ...
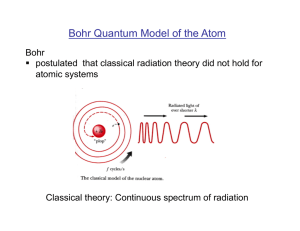
Bohr Quantum Model of the Atom
... § postulated that the electron orbital momentum is quantized Justification of Bohr’s postulates: comparison with experimental observations! ...
... § postulated that the electron orbital momentum is quantized Justification of Bohr’s postulates: comparison with experimental observations! ...
qp2
... they spin that they spin in opposite directions. Hence electrons keep their distance and lead to atomic sizes as we see. This amazing principle explains why matter doesn't bunch up into a small space and therefore why we (and the Universe) exist without imploding on ourselves. Riddle of disappearanc ...
... they spin that they spin in opposite directions. Hence electrons keep their distance and lead to atomic sizes as we see. This amazing principle explains why matter doesn't bunch up into a small space and therefore why we (and the Universe) exist without imploding on ourselves. Riddle of disappearanc ...
ph 2811 / 2808 - quantum mechanics
... 6. State and prove Ehernfest’s theorem 7. Solve the Schrodinger equation for a linear harmonic oscillator. Sketch the first two eigenfunctions of the system. 8. Determine the eigenvalue spectrum of angular momentum operators Jz and Jz 9. What are symmetric and antisymmetric wave functions? Show that ...
... 6. State and prove Ehernfest’s theorem 7. Solve the Schrodinger equation for a linear harmonic oscillator. Sketch the first two eigenfunctions of the system. 8. Determine the eigenvalue spectrum of angular momentum operators Jz and Jz 9. What are symmetric and antisymmetric wave functions? Show that ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 16. State and prove Ehernfest’s theorem 17. Solve the Schrodinger equation for a linear harmonic oscillator. Sketch the first two eigenfunctions of the system. 18. Determine the eigenvalue spectrum of angular momentum operators Jz and Jz 19. What are symmetric and antisymmetric wave functions? Show ...
... 16. State and prove Ehernfest’s theorem 17. Solve the Schrodinger equation for a linear harmonic oscillator. Sketch the first two eigenfunctions of the system. 18. Determine the eigenvalue spectrum of angular momentum operators Jz and Jz 19. What are symmetric and antisymmetric wave functions? Show ...
Quantum Theory of Light. Matter Waves.
... Modern and Classical Physics Classical physics treats particles and waves as different aspects of the reality. However, the physical reality arises from small-scale world of atoms and molecules, electrons and nuclei. Electrons behave as particles because they have charge and mass, but moving electr ...
... Modern and Classical Physics Classical physics treats particles and waves as different aspects of the reality. However, the physical reality arises from small-scale world of atoms and molecules, electrons and nuclei. Electrons behave as particles because they have charge and mass, but moving electr ...
Bohr–Einstein debates

The Bohr–Einstein debates were a series of public disputes about quantum mechanics between Albert Einstein and Niels Bohr. Their debates are remembered because of their importance to the philosophy of science. An account of the debates was written by Bohr in an article titled ""Discussions with Einsteinon Epistemological Problems in Atomic Physics"". Despite their differences of opinion regarding quantum mechanics, Bohr and Einstein had a mutual admiration that was to last the rest of their lives.The debates represent one of the highest points of scientific research in the first half of the twentieth century because it called attention to an element of quantum theory, quantum non-locality, which is absolutely central to our modern understanding of the physical world. The consensus view of professional physicists has been that Bohr proved victorious, and definitively established the fundamental probabilistic character of quantum measurement.