Astr 250 Notes on the Bohr Model Classical model
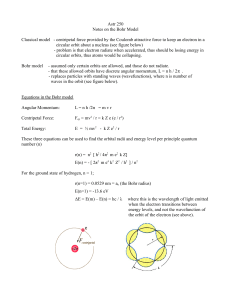
... Classical model - centripetal force provided by the Coulomb attractive force to keep an electron in a circular orbit about a nucleus (see figure below) - problem is that electron radiate when accelerated, thus should be losing energy in circular orbits, thus atoms would be collapsing. Bohr model ...
... Classical model - centripetal force provided by the Coulomb attractive force to keep an electron in a circular orbit about a nucleus (see figure below) - problem is that electron radiate when accelerated, thus should be losing energy in circular orbits, thus atoms would be collapsing. Bohr model ...
Radiation and quantised orbits
... The problem here is that there are two distinct areas of physics. The classical one and the quantum mechanical one. What goes on in the atom is essentially quantum mechanical. The laws of classical physics simply do not fully explain what is going on there. Think of the electron in orbit, in a class ...
... The problem here is that there are two distinct areas of physics. The classical one and the quantum mechanical one. What goes on in the atom is essentially quantum mechanical. The laws of classical physics simply do not fully explain what is going on there. Think of the electron in orbit, in a class ...
6.1.1
... wonder about and investigate nature, but they were the first to leave written records of their ideas. • They recorded ideas regarding a vast number of subjects from Astronomy to Zoology. • They conceptualized the building blocks of matter – which they called the ‘atom’ -- literally means ‘cannot be ...
... wonder about and investigate nature, but they were the first to leave written records of their ideas. • They recorded ideas regarding a vast number of subjects from Astronomy to Zoology. • They conceptualized the building blocks of matter – which they called the ‘atom’ -- literally means ‘cannot be ...
Read Notes #1 - Faculty Website Listing
... has changed. Classical Physics is DETERMINISTIC. In Classical physics it is possible at least theoretically for one to specify exactly both the position and velocity of a particle (i.e. its trajectory). This is what you did in homework problems in Introductory Physics. The Heisenberg uncertainty pri ...
... has changed. Classical Physics is DETERMINISTIC. In Classical physics it is possible at least theoretically for one to specify exactly both the position and velocity of a particle (i.e. its trajectory). This is what you did in homework problems in Introductory Physics. The Heisenberg uncertainty pri ...
PHY215: Study Guide for Introductory Quantum Mechanics Explain 1. Cathode Ray tubes, Cathode rays, and the generation of X‐rays.
... 4. What the spectrum of Hydrogen looks likes, and why this implies quantization of atomic states. 5. The significance of the Rutherford scattering experiment, performed first by Geiger and Marsden. 6. The Rutherford (classical) model of the atom, and its shortcomings. 7. The Bohr mo ...
... 4. What the spectrum of Hydrogen looks likes, and why this implies quantization of atomic states. 5. The significance of the Rutherford scattering experiment, performed first by Geiger and Marsden. 6. The Rutherford (classical) model of the atom, and its shortcomings. 7. The Bohr mo ...
Objective A - TuHS Physics Homepage
... 2. What important thing that they were observing at the time could Bohr’s atom predict? 3. Why do atoms make bright line spectra, and how is this related to the Bohr Orbits? Objective L: Bohr and de Broglie Problems: Chapter 27: 63c,e(150 eV, 0.13 nm) Questions: 1. How did de Broglie’s matter waves ...
... 2. What important thing that they were observing at the time could Bohr’s atom predict? 3. Why do atoms make bright line spectra, and how is this related to the Bohr Orbits? Objective L: Bohr and de Broglie Problems: Chapter 27: 63c,e(150 eV, 0.13 nm) Questions: 1. How did de Broglie’s matter waves ...
Exam topics-- understand and be able to apply ideas like in
... 14. doing multiple measurements on spin states, very basic idea of EPR experiment (whether it was right or not) 15. Notion of determined but hidden parameters (e.g. spin) or indeterminant. 16. superposition of energy states and how gives time dependence to probability(x). 17. What is the EPR paradox ...
... 14. doing multiple measurements on spin states, very basic idea of EPR experiment (whether it was right or not) 15. Notion of determined but hidden parameters (e.g. spin) or indeterminant. 16. superposition of energy states and how gives time dependence to probability(x). 17. What is the EPR paradox ...
Microsoft PowerPoint
... supported Plank-Einstein’s theorem (1923), so photons and phonons are particle like, or “real” waves (with zero static mass, reflecting the interaction between matters) can be particle like – Davison and Germer’s electron-Nickel crystal scattering experiment supported Bohr-de-Broglie’s theorem (1927 ...
... supported Plank-Einstein’s theorem (1923), so photons and phonons are particle like, or “real” waves (with zero static mass, reflecting the interaction between matters) can be particle like – Davison and Germer’s electron-Nickel crystal scattering experiment supported Bohr-de-Broglie’s theorem (1927 ...
Please look over the following review questions
... quite different from the radius predicted by Bohr that agrees with the orbital radius of Bohr ...
... quite different from the radius predicted by Bohr that agrees with the orbital radius of Bohr ...
Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... uncertainty as the photon (because of momentum conservation). As the photons that we see come from an angle of θ the photon transverse momentum uncertainty is: ∆px = p sinθ = sinθ h/λ ...
... uncertainty as the photon (because of momentum conservation). As the photons that we see come from an angle of θ the photon transverse momentum uncertainty is: ∆px = p sinθ = sinθ h/λ ...
Physical Chemistry II Review Set 1
... d. The integral of the wave function over "all space" = 1. 8. For a particle in a box of length 1nm: a. Sketch the ground state. b. Sketch the 3rd excited state. c. Using the principals of calculus, state qualitatively what you know about the derivative of the probability density where it is maximum ...
... d. The integral of the wave function over "all space" = 1. 8. For a particle in a box of length 1nm: a. Sketch the ground state. b. Sketch the 3rd excited state. c. Using the principals of calculus, state qualitatively what you know about the derivative of the probability density where it is maximum ...
453 Introduction to Quantum Mechanics (Winter 2005)
... 5. Suppose you had three particles in a one-dimensional harmonic oscillator potential, in thermal equilibrium, with total energy E = (9/2)h̄ω. If they are distinguishable particles (but all with the same mass),( i) what are the possible occupationnumber configurations? (ii) What is the most probable ...
... 5. Suppose you had three particles in a one-dimensional harmonic oscillator potential, in thermal equilibrium, with total energy E = (9/2)h̄ω. If they are distinguishable particles (but all with the same mass),( i) what are the possible occupationnumber configurations? (ii) What is the most probable ...
Homework Set 1
... An operator  is Hermitian on a space of functions F if (i) the operator  has real eigenvalues, and (ii) if operating  on functions in F gives back functions in F. For the problem below, we shall consider F to comprise all smooth, real and bounded functions in 1d space. (Bounded simply means th ...
... An operator  is Hermitian on a space of functions F if (i) the operator  has real eigenvalues, and (ii) if operating  on functions in F gives back functions in F. For the problem below, we shall consider F to comprise all smooth, real and bounded functions in 1d space. (Bounded simply means th ...
File
... quantum mechanics earned him the 1929 Nobel Prize in Physics. His doctoral thesis, which proposed that all particles have a characteristic wavelength dependent on their momentum, was so groundbreaking that the reviewers passed it directly to Einstein, who endorsed it. In opposition to the probabilis ...
... quantum mechanics earned him the 1929 Nobel Prize in Physics. His doctoral thesis, which proposed that all particles have a characteristic wavelength dependent on their momentum, was so groundbreaking that the reviewers passed it directly to Einstein, who endorsed it. In opposition to the probabilis ...
Bohr–Einstein debates

The Bohr–Einstein debates were a series of public disputes about quantum mechanics between Albert Einstein and Niels Bohr. Their debates are remembered because of their importance to the philosophy of science. An account of the debates was written by Bohr in an article titled ""Discussions with Einsteinon Epistemological Problems in Atomic Physics"". Despite their differences of opinion regarding quantum mechanics, Bohr and Einstein had a mutual admiration that was to last the rest of their lives.The debates represent one of the highest points of scientific research in the first half of the twentieth century because it called attention to an element of quantum theory, quantum non-locality, which is absolutely central to our modern understanding of the physical world. The consensus view of professional physicists has been that Bohr proved victorious, and definitively established the fundamental probabilistic character of quantum measurement.