Silicone Heat Transfer Compound
... reliable thermal coupling of electrical and electronic components is required or between any surface where thermal conductivity of heat dissipation is important. A full range of heat transfer products are available from Electrolube. This range includes non-silicone based pastes (HTC), a RTV rubber ( ...
... reliable thermal coupling of electrical and electronic components is required or between any surface where thermal conductivity of heat dissipation is important. A full range of heat transfer products are available from Electrolube. This range includes non-silicone based pastes (HTC), a RTV rubber ( ...
Test 3
... Heating & Cooling Curves (Change of Phase Diagrams) Where does PE change? Where does KE change? Where are the phase changes? Where is it solid liquid, gas? The Heat Equations Measuring Heat Energy when temp. changes ...
... Heating & Cooling Curves (Change of Phase Diagrams) Where does PE change? Where does KE change? Where are the phase changes? Where is it solid liquid, gas? The Heat Equations Measuring Heat Energy when temp. changes ...
ch 40: an introduction to animal structure and function
... adjust to environmental temperature changes. They also have low metabolic rates so the heat they generate would be too like to regulate their body temperature 2. Endotherms use metabolic heat generated by chemical reactions within their bodies to regulate their body temperatures. This is a very ener ...
... adjust to environmental temperature changes. They also have low metabolic rates so the heat they generate would be too like to regulate their body temperature 2. Endotherms use metabolic heat generated by chemical reactions within their bodies to regulate their body temperatures. This is a very ener ...
ExamView - sample-Questions-ch10-11-12
... 5. A hot (70C) lump of metal has a mass of 250 g and a specific heat of 0.25 cal/gC. John drops the metal into a 500-g calorimeter containing 75 g of water at 20C. The calorimeter is constructed of a material that has a specific heat of 0.10 cal/ gC. When equilibrium is reached, what will be t ...
... 5. A hot (70C) lump of metal has a mass of 250 g and a specific heat of 0.25 cal/gC. John drops the metal into a 500-g calorimeter containing 75 g of water at 20C. The calorimeter is constructed of a material that has a specific heat of 0.10 cal/ gC. When equilibrium is reached, what will be t ...
heated bar method
... The heated bar method is based on the theory of extended surfaces (fins). This apparatus can be used to calculate either the thermal conductivity of a material given the convection coefficient, h; or, h can be calculated given the thermal conductivity, k. The apparatus consists of a sample with rect ...
... The heated bar method is based on the theory of extended surfaces (fins). This apparatus can be used to calculate either the thermal conductivity of a material given the convection coefficient, h; or, h can be calculated given the thermal conductivity, k. The apparatus consists of a sample with rect ...
1-14 The filament of a 150 W incandescent lamp is 5 cm long and
... 1-105 A 1000-W iron is left on the iron board with its base exposed to the air at 20°C. The temperature of the base of the iron is to be determined in steady operation. Assumptions 1 Steady operating conditions exist. 2 The thermal properties of the iron base and the convection heat transfer coeffi ...
... 1-105 A 1000-W iron is left on the iron board with its base exposed to the air at 20°C. The temperature of the base of the iron is to be determined in steady operation. Assumptions 1 Steady operating conditions exist. 2 The thermal properties of the iron base and the convection heat transfer coeffi ...
HOMEWORK #2
... from the refridgerator and becomes warmer, while the refridgerator gives up 10 J of energy and becomes colder. Would this energy transfer violate the first law of thermodynamics? Would this energy transfer violate the second law of thermodynamics? Explain. ...
... from the refridgerator and becomes warmer, while the refridgerator gives up 10 J of energy and becomes colder. Would this energy transfer violate the first law of thermodynamics? Would this energy transfer violate the second law of thermodynamics? Explain. ...
Energy Content of Food
... system as follows: A calorie is the amount of energy required to raise 1 gram of water 1 degree Celsius. Click on the food to go to a calorie calculator. ...
... system as follows: A calorie is the amount of energy required to raise 1 gram of water 1 degree Celsius. Click on the food to go to a calorie calculator. ...
Honors Chemistry Quiz Chapter 6: Thermochemistry - Doc-U-Ment
... This quiz is worth 40 points; each correct response is 2 points. Only those quizzes completed in black ink will be graded. Good luck! ...
... This quiz is worth 40 points; each correct response is 2 points. Only those quizzes completed in black ink will be graded. Good luck! ...
Unit 6 Review
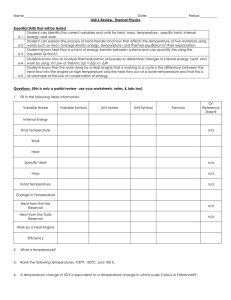
... Students know how to analyze thermodynamic processes to determine changes in internal energy, heat, and 6-4 work by using 1st Law of Thermo: ΔU = ΔQ +/- ΔW Students know that the work done by a heat engine that is working in a cycle is the difference between the heat flow into the engine at high tem ...
... Students know how to analyze thermodynamic processes to determine changes in internal energy, heat, and 6-4 work by using 1st Law of Thermo: ΔU = ΔQ +/- ΔW Students know that the work done by a heat engine that is working in a cycle is the difference between the heat flow into the engine at high tem ...
Tarea III
... 6–76 In tropical climates, the water near the surface of the ocean remains warm throughout the year as a result of solar energy absorption. In the deeper parts of the ocean, however, the water remains at a relatively low temperature since the sun’s rays cannot penetrate very far. It is proposed to ...
... 6–76 In tropical climates, the water near the surface of the ocean remains warm throughout the year as a result of solar energy absorption. In the deeper parts of the ocean, however, the water remains at a relatively low temperature since the sun’s rays cannot penetrate very far. It is proposed to ...
physics of foil - P1 International
... PHYSICAL CONTACT of one part of the same body with another part, or of one body with another. For instance, if one end of an iron rod is heat, the heat travels by conduction through the metal to the other end; it also travels to the surface and is conducted to the surrounding air which is another, b ...
... PHYSICAL CONTACT of one part of the same body with another part, or of one body with another. For instance, if one end of an iron rod is heat, the heat travels by conduction through the metal to the other end; it also travels to the surface and is conducted to the surrounding air which is another, b ...
Process Heat Transfer Lab - University of Engineering and Technology
... Heat transfer by simultaneous conduction and convection whether free or forced forms the basis of most industrial heat exchangers and related equipment. Using the instrumentation provided, free and forced convective heat transfer coefficients may be determined for, a flat surface, an array of cylind ...
... Heat transfer by simultaneous conduction and convection whether free or forced forms the basis of most industrial heat exchangers and related equipment. Using the instrumentation provided, free and forced convective heat transfer coefficients may be determined for, a flat surface, an array of cylind ...
Skills Worksheet
... through a passage around a fire. Once heated by the fire, the warm air flowed back into the room. In the 1760s, James Watt experimented with steam engines and made substantial improvements to their design. Within 100 years of his experiments, central heating with steam was widely used in schools, ch ...
... through a passage around a fire. Once heated by the fire, the warm air flowed back into the room. In the 1760s, James Watt experimented with steam engines and made substantial improvements to their design. Within 100 years of his experiments, central heating with steam was widely used in schools, ch ...
Specific Heat of a Metal
... Chemists identify substances on the basis of their chemical and physical properties. One physical property of a substance is the amount of energy it will absorb per unit of mass. This property can be measured quite accurately and is called specific heat (Cp). Specific heat is the amount of energy, m ...
... Chemists identify substances on the basis of their chemical and physical properties. One physical property of a substance is the amount of energy it will absorb per unit of mass. This property can be measured quite accurately and is called specific heat (Cp). Specific heat is the amount of energy, m ...
First law of thermodynamics
... Volume is gradually doubled with no heat input, then heated at constant volume to the initial temperature. System is heated at constant pressure until volume doubles, then cooled at constant volume to the initial temperature. System is allowed to expand into a vacuum (free expansion) to twice its vo ...
... Volume is gradually doubled with no heat input, then heated at constant volume to the initial temperature. System is heated at constant pressure until volume doubles, then cooled at constant volume to the initial temperature. System is allowed to expand into a vacuum (free expansion) to twice its vo ...
p250c13
... Engine Efficiency (in general) V net mechanical work comes from net transfer of heat W = QH QC Efficiency is the effectiveness with which supplied heat QH is converted to work :For the QC W QH QC Eff ...
... Engine Efficiency (in general) V net mechanical work comes from net transfer of heat W = QH QC Efficiency is the effectiveness with which supplied heat QH is converted to work :For the QC W QH QC Eff ...
Werribee Centrals FNC in conjunction with AFL AND
... The Football Body should comply with AS1768-2007, entitled The Lightning Protection Standard, published on 10 January 2007 (Lightning Standard). While the Lightning Standard will not necessarily prevent damage or personal injury due to lightning, it will reduce the probability of such damage or inju ...
... The Football Body should comply with AS1768-2007, entitled The Lightning Protection Standard, published on 10 January 2007 (Lightning Standard). While the Lightning Standard will not necessarily prevent damage or personal injury due to lightning, it will reduce the probability of such damage or inju ...
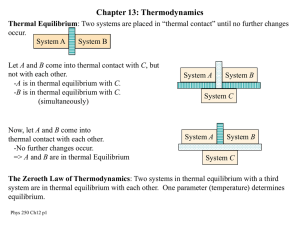
Chap #13
... associated with scuffing your feet acting through the lengths of the scuffs does mechanical work. The first law of thermodynamics tells us that this work is just as effective in raising the temperature of our feet as an equivalent amount of heat. Example: Imagine that you are sitting in a bathtub wi ...
... associated with scuffing your feet acting through the lengths of the scuffs does mechanical work. The first law of thermodynamics tells us that this work is just as effective in raising the temperature of our feet as an equivalent amount of heat. Example: Imagine that you are sitting in a bathtub wi ...
cd-79f-2-series narrow differential thermostats
... When installing the thermostat, good heat transfer must be insured. The heat sensitive side of the switch (the base) should be positioned on the heat source. Heat-conducting paste or lacquer improves heat transfer. Please note that in the standard version the thermostat has an electrically live hous ...
... When installing the thermostat, good heat transfer must be insured. The heat sensitive side of the switch (the base) should be positioned on the heat source. Heat-conducting paste or lacquer improves heat transfer. Please note that in the standard version the thermostat has an electrically live hous ...
09-TempControls
... • Heat: the transfer of energy from one object to another flows from higher to lower temp • Three main types of energy transfer: • Convection • Conduction • Radiation ...
... • Heat: the transfer of energy from one object to another flows from higher to lower temp • Three main types of energy transfer: • Convection • Conduction • Radiation ...
Heat stress round table
... implement a specific strategy during the carry over period? • What is, from your point of view, the main limiting factor to improve conception rate during the heat stress period? ...
... implement a specific strategy during the carry over period? • What is, from your point of view, the main limiting factor to improve conception rate during the heat stress period? ...
11-Heat Energy
... Specific Heat Capacity Is the heat capacity per unit mass of a material. It does not depend on how large the object is – only what it is made of. Its name is often shortened to just “specific heat”. ...
... Specific Heat Capacity Is the heat capacity per unit mass of a material. It does not depend on how large the object is – only what it is made of. Its name is often shortened to just “specific heat”. ...
Hyperthermia
Hyperthermia is elevated body temperature due to failed thermoregulation that occurs when a body produces or absorbs more heat than it dissipates. Extreme temperature elevation then becomes a medical emergency requiring immediate treatment to prevent disability or death.The most common causes include heat stroke and adverse reactions to drugs. The former is an acute temperature elevation caused by exposure to excessive heat, or combination of heat and humidity, that overwhelms the heat-regulating mechanisms. The latter is a relatively rare side effect of many drugs, particularly those that affect the central nervous system. Malignant hyperthermia is a rare complication of some types of general anesthesia.Hyperthermia differs from fever in that the body's temperature set point remains unchanged. The opposite is hypothermia, which occurs when the temperature drops below that required to maintain normal metabolism.