Radiation
... the ocean water, but each does so in a different way. The sand absorbs energy more quickly while bodies of water absorb it more slowly. This explains why the sand is extremely hot at noon while the ocean water is relatively cool. Heat is a form of energy that an object has when its atoms and molecul ...
... the ocean water, but each does so in a different way. The sand absorbs energy more quickly while bodies of water absorb it more slowly. This explains why the sand is extremely hot at noon while the ocean water is relatively cool. Heat is a form of energy that an object has when its atoms and molecul ...
Specific Heat of a Metal
... temperature. The heat gained by the cooler substance equals the heat lost by the warmer substance, if we assume no loss of heat to the surrounding environment. Heat lost = Heat gained In this experiment, you will determine the specific heat constant of a metal. The metal sample will be heated to a h ...
... temperature. The heat gained by the cooler substance equals the heat lost by the warmer substance, if we assume no loss of heat to the surrounding environment. Heat lost = Heat gained In this experiment, you will determine the specific heat constant of a metal. The metal sample will be heated to a h ...
World Biomes - Tartu Veeriku Kool
... • Dry desert climates are formed by highpressure zones in which cold air descends. Then the descending air becomes warm but, instead of releasing rain, the heat from the ground evaporates the water before it can come down as rain. The ground is super hot because the sun's rays beat down on it direct ...
... • Dry desert climates are formed by highpressure zones in which cold air descends. Then the descending air becomes warm but, instead of releasing rain, the heat from the ground evaporates the water before it can come down as rain. The ground is super hot because the sun's rays beat down on it direct ...
Calorimetry and Specific Heat
... of 10 grams of water by one degree C? Answer: 10 calories • How much heat is needed to raise the temperature of 10 grams of water by 10 degrees C? • Answer: 100 calories ...
... of 10 grams of water by one degree C? Answer: 10 calories • How much heat is needed to raise the temperature of 10 grams of water by 10 degrees C? • Answer: 100 calories ...
what to know about meteorology list
... 1. Air moves clockwise and outward from a high. (“High to Low”) (In the northern hemisphere) 2. Air moves counter-clockwise and into a low (“High to Low”) (In the northern hemisphere) 3. Remember: “The flow will go from high to low”. 4. As moisture increases, air pressure decreases (“wet” air is les ...
... 1. Air moves clockwise and outward from a high. (“High to Low”) (In the northern hemisphere) 2. Air moves counter-clockwise and into a low (“High to Low”) (In the northern hemisphere) 3. Remember: “The flow will go from high to low”. 4. As moisture increases, air pressure decreases (“wet” air is les ...
Thermochemistry PPT
... • Energy – capacity for doing work or supplying heat. • Thermochemistry – study of energy changes that occur during phase changes and chem. rxns. • Chem. Potential Energy – energy stored in chemical bonds. ...
... • Energy – capacity for doing work or supplying heat. • Thermochemistry – study of energy changes that occur during phase changes and chem. rxns. • Chem. Potential Energy – energy stored in chemical bonds. ...
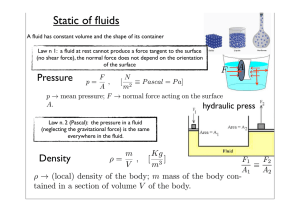
Static of fluids
... (in which there are not phase transitions); ∆T variation of temperature; m mass of the body; c [cal / Kg o C] specific heat; C [cal / o C] heat capacity of the body In purely thermal transformations the heat is conserved Heat (latent) of transition A = Lm, where L is the specific latent heat ...
... (in which there are not phase transitions); ∆T variation of temperature; m mass of the body; c [cal / Kg o C] specific heat; C [cal / o C] heat capacity of the body In purely thermal transformations the heat is conserved Heat (latent) of transition A = Lm, where L is the specific latent heat ...
Teacher`s notes 22 Specific Heat Capacity of a solid
... this reason it is best to purchase a block and heater that are matched. Ideally several blocks are required, aluminium, brass and iron. Glycerol is mentioned as the heat conductive medium to surround the heater this reduces the risk or fire. Oil with a very high boiling point and flash point can be ...
... this reason it is best to purchase a block and heater that are matched. Ideally several blocks are required, aluminium, brass and iron. Glycerol is mentioned as the heat conductive medium to surround the heater this reduces the risk or fire. Oil with a very high boiling point and flash point can be ...
Heat on the move
... Heat can move from one place to another – heat Put the three spoons into the container of hot is transferred. It always moves from somewhere water. Feel the ends of the spoons. Which of the or something hot to a place or object that’s cooler. ends became hot? Can you think of some examples? When hea ...
... Heat can move from one place to another – heat Put the three spoons into the container of hot is transferred. It always moves from somewhere water. Feel the ends of the spoons. Which of the or something hot to a place or object that’s cooler. ends became hot? Can you think of some examples? When hea ...
Heat And Thermodynamics - Figure B
... Heat is a form of energy which appears when two bodies at different temperature are placed into thermal contact. It can flow from high temperature to low temperature till temperature of the two bodies becomes same. Thus, we can say that heat is the energy in transit. Heat is not property of system, ...
... Heat is a form of energy which appears when two bodies at different temperature are placed into thermal contact. It can flow from high temperature to low temperature till temperature of the two bodies becomes same. Thus, we can say that heat is the energy in transit. Heat is not property of system, ...
Specific Heat
... Learning Check 2. Two objects are sitting next to each other in the sunlight. Object A gets hotter than object B. A. Object A has a lower specific heat than object B B. Object A has a higher specific heat than object B C. Both objects have the same specific heat ...
... Learning Check 2. Two objects are sitting next to each other in the sunlight. Object A gets hotter than object B. A. Object A has a lower specific heat than object B B. Object A has a higher specific heat than object B C. Both objects have the same specific heat ...
Heat Transfer conduction
... Heat energy is transferred from a high heat “source” to a low heat “sink”. Heat energy will “flow” from high temperature areas to low temperature ones through one of three methods; radiation, convection or conduction. Radiation is a mode of energy transfer that does not require a medium, or substanc ...
... Heat energy is transferred from a high heat “source” to a low heat “sink”. Heat energy will “flow” from high temperature areas to low temperature ones through one of three methods; radiation, convection or conduction. Radiation is a mode of energy transfer that does not require a medium, or substanc ...
Note: Moving air
... Question: Describe all the heat transfer steps in heating water on a stove. Answer: A) Heat is conducted from the heating element or gas flame into the bottom of the pan. B) The water next to the bottom of the pan is heated by conduction. C) The heated water rises by convection and is replaced by co ...
... Question: Describe all the heat transfer steps in heating water on a stove. Answer: A) Heat is conducted from the heating element or gas flame into the bottom of the pan. B) The water next to the bottom of the pan is heated by conduction. C) The heated water rises by convection and is replaced by co ...
Nervous system: Detects information from the environment
... Protects the body's internal living tissues and organs against: invasion by infectious organisms dehydration abrupt changes in temperature Disposes of waste materials Contains receptors for touch, pressure, pain, heat, and cold. Stores water and fat. Provides shape and support Allows you to move Pro ...
... Protects the body's internal living tissues and organs against: invasion by infectious organisms dehydration abrupt changes in temperature Disposes of waste materials Contains receptors for touch, pressure, pain, heat, and cold. Stores water and fat. Provides shape and support Allows you to move Pro ...
Heat transfer - hrsbstaff.ednet.ns.ca
... to shiver. You grab your towel, dry off, and lay in the sun again, feeling comfortable. How is solar energy interacting with air, land, and water? ...
... to shiver. You grab your towel, dry off, and lay in the sun again, feeling comfortable. How is solar energy interacting with air, land, and water? ...
Maintaining a Constant Internal Environment
... Enzymes: Rxn rates inc. 2-3 times with each 100 C temp. inc. (until denatured) Each species has an optimal temp. range for metabolic rxns to be efficient Thermoregulation Organisms maintain their body temp within optimal range (various methods) ...
... Enzymes: Rxn rates inc. 2-3 times with each 100 C temp. inc. (until denatured) Each species has an optimal temp. range for metabolic rxns to be efficient Thermoregulation Organisms maintain their body temp within optimal range (various methods) ...
Implimenting a Simple Heat Exchanger Unit with
... any temperature difference between the hot side and the air adds to the overall temperature difference, which reduces the efficiency of the system. It is thus advantageous to use the maximum cooling potential available since this translates directly to a more efficient system, where ...
... any temperature difference between the hot side and the air adds to the overall temperature difference, which reduces the efficiency of the system. It is thus advantageous to use the maximum cooling potential available since this translates directly to a more efficient system, where ...
SC151 - CHAPTER 9 LEARNING OBJECTIVES
... Demonstrate an understanding of thermochemistry by: • explaining the relationships among the following: system, surroundings, and universe; exothermic process and endothermic process; internal energy (E) and enthalpy (H); ∆E, ∆H, qv, and qp • sketching an energy diagram such as those shown in Figure ...
... Demonstrate an understanding of thermochemistry by: • explaining the relationships among the following: system, surroundings, and universe; exothermic process and endothermic process; internal energy (E) and enthalpy (H); ∆E, ∆H, qv, and qp • sketching an energy diagram such as those shown in Figure ...
AA2 FALL 2005
... Conduction is the process through which heat is diffused to cooler materials as radiation is absorbed. Land surfaces heat quickly, while water bodies can mix and have higher heat capacity. Solids (land) are better conductors than gases (atmosphere). Convection is physical mixing with a strong vertic ...
... Conduction is the process through which heat is diffused to cooler materials as radiation is absorbed. Land surfaces heat quickly, while water bodies can mix and have higher heat capacity. Solids (land) are better conductors than gases (atmosphere). Convection is physical mixing with a strong vertic ...
Microclimatology 2 FALL 2008
... Conduction is the process through which heat is diffused to cooler materials as radiation is absorbed. Land surfaces heat quickly, while water bodies can mix and have higher heat capacity. Solids (land) are better conductors than gases (atmosphere). Convection is physical mixing with a strong vertic ...
... Conduction is the process through which heat is diffused to cooler materials as radiation is absorbed. Land surfaces heat quickly, while water bodies can mix and have higher heat capacity. Solids (land) are better conductors than gases (atmosphere). Convection is physical mixing with a strong vertic ...
Comparison of Heat Loss by Sample Building Component
... Comparison of Heat Loss by Sample Building Component Windows vs. Walls Formula: Heat Loss (BTU/hr) = UA Where U = Thermal transmittance Where A = Area Where = Delta T (temperature difference) Use the formula for calculating heat loss to discuss window replacement as an energy savings measure. Use th ...
... Comparison of Heat Loss by Sample Building Component Windows vs. Walls Formula: Heat Loss (BTU/hr) = UA Where U = Thermal transmittance Where A = Area Where = Delta T (temperature difference) Use the formula for calculating heat loss to discuss window replacement as an energy savings measure. Use th ...
Chapter 15 – Section 2 Heat
... Heat and Thermal Energy • Heat is the transfer of thermal energy from one object to another when the objects are at different temperatures. • The amount of heat that is transferred when two objects are brought into contact depends on the difference in temperature between the objects. ...
... Heat and Thermal Energy • Heat is the transfer of thermal energy from one object to another when the objects are at different temperatures. • The amount of heat that is transferred when two objects are brought into contact depends on the difference in temperature between the objects. ...
What is the DSC used for
... sensor or between the sensor and sample pan. The cell sensor consists of a constantan body with separate raised platforms or vessel to hold the sample and reference (Fig. 1). The platforms are connected to the heating block (base) by thin-walled tubes that create thermal resistances between the plat ...
... sensor or between the sensor and sample pan. The cell sensor consists of a constantan body with separate raised platforms or vessel to hold the sample and reference (Fig. 1). The platforms are connected to the heating block (base) by thin-walled tubes that create thermal resistances between the plat ...
What is the DSC used for
... sensor or between the sensor and sample pan. The cell sensor consists of a constantan body with separate raised platforms or vessel to hold the sample and reference (Fig. 1). The platforms are connected to the heating block (base) by thin-walled tubes that create thermal resistances between the plat ...
... sensor or between the sensor and sample pan. The cell sensor consists of a constantan body with separate raised platforms or vessel to hold the sample and reference (Fig. 1). The platforms are connected to the heating block (base) by thin-walled tubes that create thermal resistances between the plat ...
Phase Changes and latent heat
... freeze or condense (gas to liquid). The energy can be heat transfer or can be due to work done on or byy the system. y Energy used to cause a phase change does not cause a temperature change. When ice melts at OoC it becomes water t att 0oC; C when h water t b boils il att 100oC,i Citb becomes steam ...
... freeze or condense (gas to liquid). The energy can be heat transfer or can be due to work done on or byy the system. y Energy used to cause a phase change does not cause a temperature change. When ice melts at OoC it becomes water t att 0oC; C when h water t b boils il att 100oC,i Citb becomes steam ...
Hyperthermia
Hyperthermia is elevated body temperature due to failed thermoregulation that occurs when a body produces or absorbs more heat than it dissipates. Extreme temperature elevation then becomes a medical emergency requiring immediate treatment to prevent disability or death.The most common causes include heat stroke and adverse reactions to drugs. The former is an acute temperature elevation caused by exposure to excessive heat, or combination of heat and humidity, that overwhelms the heat-regulating mechanisms. The latter is a relatively rare side effect of many drugs, particularly those that affect the central nervous system. Malignant hyperthermia is a rare complication of some types of general anesthesia.Hyperthermia differs from fever in that the body's temperature set point remains unchanged. The opposite is hypothermia, which occurs when the temperature drops below that required to maintain normal metabolism.