Energy - Montana State University Billings
... surface and transferring heat to your skin – The warmer it is, the faster molecules move in a random fashion and the more collisions with your skin per unit time – Could you feel warm in a place where the temperature is low? ...
... surface and transferring heat to your skin – The warmer it is, the faster molecules move in a random fashion and the more collisions with your skin per unit time – Could you feel warm in a place where the temperature is low? ...
INTRODUCTION TO HUMAN BIOLOGY pp. 907-910
... • There is a rumor that a bank is going out of business. People get scared and start going to the bank to take out their money. People see that the bank is getting weak because of all the people taking out their money so they go to take their money. This happens more and more until the bank has no m ...
... • There is a rumor that a bank is going out of business. People get scared and start going to the bank to take out their money. People see that the bank is getting weak because of all the people taking out their money so they go to take their money. This happens more and more until the bank has no m ...
nupoc study guide - UC Berkeley NROTC
... super-heated slightly above the saturation temperature. The increase in temperature over time can be expressed graphically as shown below. T Tsat ...
... super-heated slightly above the saturation temperature. The increase in temperature over time can be expressed graphically as shown below. T Tsat ...
Specific Heat Lab Experiment Sixteen p
... Purpose: To determine the identity of an unknown metal by determining its specific heat capacity, “c” Materials: unknown metal sample, calorimeter, styrofoam cup, water, beaker, hot plate, string, thermometer, balance Background: If substances of different temperatures are in contact with each other ...
... Purpose: To determine the identity of an unknown metal by determining its specific heat capacity, “c” Materials: unknown metal sample, calorimeter, styrofoam cup, water, beaker, hot plate, string, thermometer, balance Background: If substances of different temperatures are in contact with each other ...
L 17
... another because of their temperature difference. • Heat stops flowing when the two systems come to the same temperature. • Heat was considered to be an actual fluid (caloric), but it is NOT a fluid- it is energy! ...
... another because of their temperature difference. • Heat stops flowing when the two systems come to the same temperature. • Heat was considered to be an actual fluid (caloric), but it is NOT a fluid- it is energy! ...
Heat Transfer by Conduction
... For glass and most nonporous materials, the thermal conductivities are much lower, from about 0.35 to 3.5. For most liquid k is lower than that for solids, with typical values of about 0.17. k decreases by 3 ~ 4 %t for a 10 ºC rise in temperature, except water. ...
... For glass and most nonporous materials, the thermal conductivities are much lower, from about 0.35 to 3.5. For most liquid k is lower than that for solids, with typical values of about 0.17. k decreases by 3 ~ 4 %t for a 10 ºC rise in temperature, except water. ...
HEAT ENERGY
... more room if heated – this is why things expand if heated • It is also why substances change from: solids liquids gases when heated Visit www.worldofteaching.com for more free powerpoints ...
... more room if heated – this is why things expand if heated • It is also why substances change from: solids liquids gases when heated Visit www.worldofteaching.com for more free powerpoints ...
heat energy - Parkway C-2
... sea. The warm air rises over the land and cool air falls over the sea. So we feel a sea breeze. (You will talk more about this in 8th grade) Rising convection currents can be uses by glider pilots to keep their planes in the air and by birds to stay aloft. ...
... sea. The warm air rises over the land and cool air falls over the sea. So we feel a sea breeze. (You will talk more about this in 8th grade) Rising convection currents can be uses by glider pilots to keep their planes in the air and by birds to stay aloft. ...
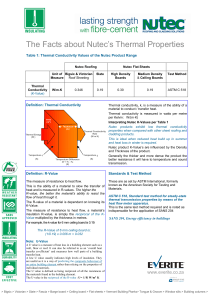
The Facts about Nutec Thermal Properties 11 5 12.pub
... thermal transmission properties by means of the heat flow meter apparatus. This is the same test method required and is noted as indispensable for the application of SANS 204 ...
... thermal transmission properties by means of the heat flow meter apparatus. This is the same test method required and is noted as indispensable for the application of SANS 204 ...
performances of flat-plate and cpc solar collectors in underfloor
... Collector (CPC) collectors to replace Flat-Plat collectors in solar energy underfloor heating systems. By this way, it is aimed to explore the feasibility of area reduction required by the collectors. Secondly, the temperature profiles of the circulating water loops and the concrete slabs are sought ...
... Collector (CPC) collectors to replace Flat-Plat collectors in solar energy underfloor heating systems. By this way, it is aimed to explore the feasibility of area reduction required by the collectors. Secondly, the temperature profiles of the circulating water loops and the concrete slabs are sought ...
The Heat Equation - Rose
... The other common type of boundary condition is the Neumann condition, in which we specify the rate at which heat energy is being pumped into the ends of the bar. Now since q = −c1 ρx = −kc1 ux quantifies the rate of heat flow, we typically specify conditions like −kc1 ux (0, t) = ψ0 (t) (the rate hea ...
... The other common type of boundary condition is the Neumann condition, in which we specify the rate at which heat energy is being pumped into the ends of the bar. Now since q = −c1 ρx = −kc1 ux quantifies the rate of heat flow, we typically specify conditions like −kc1 ux (0, t) = ψ0 (t) (the rate hea ...
Thermodynamics
... temperature increased by 3.682oc. The heat capacity of the calorimeter was 3.56 kJ/oc, and the calorimeter contained 1.00 kg of water. Find the molar heat in ...
... temperature increased by 3.682oc. The heat capacity of the calorimeter was 3.56 kJ/oc, and the calorimeter contained 1.00 kg of water. Find the molar heat in ...
Thermodynamics
... Heat is a form of energy so we can always use Joules. More common in thermodynamics is the calorie: By definition 1 calorie is the amount of heat required to change the temperature of 1 gram of water 1°C. ...
... Heat is a form of energy so we can always use Joules. More common in thermodynamics is the calorie: By definition 1 calorie is the amount of heat required to change the temperature of 1 gram of water 1°C. ...
Geology :: 3. Energy and the Dynamic Earth
... conduction. Conduction does not cause the movement of hot material from one place to another. The atoms remain in the crystalline structure and transport the heat by oscillation. In gases and liquids, heat transport take place by convection. Convection, unlike conduction, does cause movement. It is ...
... conduction. Conduction does not cause the movement of hot material from one place to another. The atoms remain in the crystalline structure and transport the heat by oscillation. In gases and liquids, heat transport take place by convection. Convection, unlike conduction, does cause movement. It is ...
Human Body Introduction
... Muscle tissue (most abundant): controls internal movements of materials (ex: blood, food) Epithelial tissue: closely packed cells covering the surface of the body and line internal organs (ex: inside chambers of heart, glands) Connective tissue: holds organs in place and binds different parts ...
... Muscle tissue (most abundant): controls internal movements of materials (ex: blood, food) Epithelial tissue: closely packed cells covering the surface of the body and line internal organs (ex: inside chambers of heart, glands) Connective tissue: holds organs in place and binds different parts ...
Heat Heat Capacity Latent Heat Latent Heat
... Did Not Cover. Will be covered in Lec 3-6 Objects that become sufficiently hot will glow visibly; as they get hotter they go from red, to yellow, to a bluish white. This is electromagnetic radiation; objects at any temperature will emit it at various frequencies, from radio waves all the way to gamm ...
... Did Not Cover. Will be covered in Lec 3-6 Objects that become sufficiently hot will glow visibly; as they get hotter they go from red, to yellow, to a bluish white. This is electromagnetic radiation; objects at any temperature will emit it at various frequencies, from radio waves all the way to gamm ...
Exam 3 review - Iowa State University
... j. None of the above 7.Nickel crystallizes in a face-centered cubic lattice. If the density of the metal is 8.908 g/cm3, what is the unit cell edge length in cm? 8.A piece of copper metal of mass 6.22 kg is heated from 20.5 °C to 324.3 °C. Calculate the heat absorbed by the metal. Specific heat is 0 ...
... j. None of the above 7.Nickel crystallizes in a face-centered cubic lattice. If the density of the metal is 8.908 g/cm3, what is the unit cell edge length in cm? 8.A piece of copper metal of mass 6.22 kg is heated from 20.5 °C to 324.3 °C. Calculate the heat absorbed by the metal. Specific heat is 0 ...
POWERPOINT SCIENCE
... The measure of the average kinetic energy of the individual particles in an object. Matter is made up of tiny particles called atoms and molecules. These particles are always in motion even if the object they make up isn’t moving at all. Energy of motion is called kinetic energy, so all particle ...
... The measure of the average kinetic energy of the individual particles in an object. Matter is made up of tiny particles called atoms and molecules. These particles are always in motion even if the object they make up isn’t moving at all. Energy of motion is called kinetic energy, so all particle ...
Project Meeting Minutes Template
... Determination of Δx o Find conductivity dependence on composition o Heating orientation (1 side vs. both sides) o From Chad: “The next step we have to do is to compare the numerical model against an analytical solution for heat transfer where we have temperature gradients inside the aluminum.” ...
... Determination of Δx o Find conductivity dependence on composition o Heating orientation (1 side vs. both sides) o From Chad: “The next step we have to do is to compare the numerical model against an analytical solution for heat transfer where we have temperature gradients inside the aluminum.” ...
W9e „Heat Capacity of Solids and Liquids“
... is given by the sum Ctot= CK+ Cfl. By measurement of the electrical power Pel UI and the determination of the slope b=T/t of the time dependent temperature rise, the heat capacity Ctot can be calculated from Ctot UI / b . The temperature is measured with a digital thermometer. The measurement ...
... is given by the sum Ctot= CK+ Cfl. By measurement of the electrical power Pel UI and the determination of the slope b=T/t of the time dependent temperature rise, the heat capacity Ctot can be calculated from Ctot UI / b . The temperature is measured with a digital thermometer. The measurement ...
TemperATures A Tale of Two pArT 1
... most cases, people can find respite by finding a place that is cool enough or by utilizing a fan to help with the evaporation and heat removal. However, as was the case in Chicago, if the combined heat and humidity is too high, then there is no way to keep cool. In other words, the air contains more ...
... most cases, people can find respite by finding a place that is cool enough or by utilizing a fan to help with the evaporation and heat removal. However, as was the case in Chicago, if the combined heat and humidity is too high, then there is no way to keep cool. In other words, the air contains more ...
Neonatal Thermoregulation
... Allen, K. (2011) Neonatal thermal care: A discussion of two incubator modes for optimising thermoregulation. A care study. Journal of Neonatal Nursing. 17, 2; 43-48 Aylott, M. (2006a) The Neonatal energy triangle part 1; Metabolic adaptation. Paediatric Nursing. 18, ...
... Allen, K. (2011) Neonatal thermal care: A discussion of two incubator modes for optimising thermoregulation. A care study. Journal of Neonatal Nursing. 17, 2; 43-48 Aylott, M. (2006a) The Neonatal energy triangle part 1; Metabolic adaptation. Paediatric Nursing. 18, ...
Hyperthermia
Hyperthermia is elevated body temperature due to failed thermoregulation that occurs when a body produces or absorbs more heat than it dissipates. Extreme temperature elevation then becomes a medical emergency requiring immediate treatment to prevent disability or death.The most common causes include heat stroke and adverse reactions to drugs. The former is an acute temperature elevation caused by exposure to excessive heat, or combination of heat and humidity, that overwhelms the heat-regulating mechanisms. The latter is a relatively rare side effect of many drugs, particularly those that affect the central nervous system. Malignant hyperthermia is a rare complication of some types of general anesthesia.Hyperthermia differs from fever in that the body's temperature set point remains unchanged. The opposite is hypothermia, which occurs when the temperature drops below that required to maintain normal metabolism.