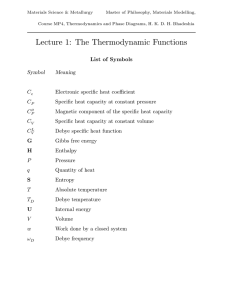
Thermodynamic functions - Phase Transformations Group
... The Helmholtz free energy F is the corresponding term at constant volume, when H is replaced by U in equation 9. A process can occur spontaneously if it leads to a reduction in the free energy. Quantities such as H, G and S are path independent and therefore are called functions of state. More About ...
... The Helmholtz free energy F is the corresponding term at constant volume, when H is replaced by U in equation 9. A process can occur spontaneously if it leads to a reduction in the free energy. Quantities such as H, G and S are path independent and therefore are called functions of state. More About ...
Chapter Two Atoms & The Periodic Table
... decomposing 1 mole of sulfur trioxide gas into sulfur dioxide gas and oxygen gas? (Hint: use properties of enthalpy!) ...
... decomposing 1 mole of sulfur trioxide gas into sulfur dioxide gas and oxygen gas? (Hint: use properties of enthalpy!) ...
File - Ms. A Science Online
... Radiated heat energy travels through empty space. Electromagnetic waves travel at the speed of light, which is 300,000,000 meters per second. Sometimes these waves are visible, like when something is “red hot.” You can see how hot it is, but you can also feel it from a distance, as your skin absorbs ...
... Radiated heat energy travels through empty space. Electromagnetic waves travel at the speed of light, which is 300,000,000 meters per second. Sometimes these waves are visible, like when something is “red hot.” You can see how hot it is, but you can also feel it from a distance, as your skin absorbs ...
Honors Biology - Honors Class Help
... 3. Positive feedback - the results of a process intensifies that same process Ex: labor - muscles contract uterus, uterus stimulates release of more hormones, more contractions. “On and on more switch” 3. Thermoregulation - the maintenance of internal body temperature close to a set point. ...
... 3. Positive feedback - the results of a process intensifies that same process Ex: labor - muscles contract uterus, uterus stimulates release of more hormones, more contractions. “On and on more switch” 3. Thermoregulation - the maintenance of internal body temperature close to a set point. ...
Real People Doing Real Science
... thorax and abdomen is not an important mechanism for regulating body temperature in flying honeybees. So how do honeybees keep cool while flying? In stationary animals including birds, variation in metabolic heat production is an important mechanism of thermoregulation (witness shivering in response ...
... thorax and abdomen is not an important mechanism for regulating body temperature in flying honeybees. So how do honeybees keep cool while flying? In stationary animals including birds, variation in metabolic heat production is an important mechanism of thermoregulation (witness shivering in response ...
homeostasis and feedback with video clip
... • Sensors – structures including receptors on target cells that will monitor levels relative to set point • Controller – determine if levels are within set point range and what response is necessary to return to range • Effector – parts of body that physically make the change to return to set point ...
... • Sensors – structures including receptors on target cells that will monitor levels relative to set point • Controller – determine if levels are within set point range and what response is necessary to return to range • Effector – parts of body that physically make the change to return to set point ...
Measuring the Specific Heat Capacity of Water
... Energy for 1˚C temperature rise = total energy used ÷ 15 = _____________ ÷ 15 = ________ J ...
... Energy for 1˚C temperature rise = total energy used ÷ 15 = _____________ ÷ 15 = ________ J ...
Thermochemistry Energy - the capacity to do work Potential energy
... open system - exchanges matter and energy with the surroundings closed system - exchanges energy but not matter with the surroundings isolated system - exchanges neither energy nor matter with the surroundings Internal Energy (U) - the total energy contained within the system Heat (q) - the energy t ...
... open system - exchanges matter and energy with the surroundings closed system - exchanges energy but not matter with the surroundings isolated system - exchanges neither energy nor matter with the surroundings Internal Energy (U) - the total energy contained within the system Heat (q) - the energy t ...
Temperature Coefficient of Resistance
... that is the temperature coefficient of resistivity. If this behavior were actually a control designed by man, what would have driven him to dream up such an idea? The temperature of the conductor presents a problem in that once it reaches a critical point, the atomic bonds of the conductor will brea ...
... that is the temperature coefficient of resistivity. If this behavior were actually a control designed by man, what would have driven him to dream up such an idea? The temperature of the conductor presents a problem in that once it reaches a critical point, the atomic bonds of the conductor will brea ...
Chapter_7_Energy_and_Phase_Changes_REVISED 2
... Note: specific heat of water is 4.18 joules/gramC (which means that it takes 4.18 joules to raise one gram of water 1 C) Ex: How much heat energy in joules is absorbed by 30 grams of water (H2O) when it is heated from 20C to 30C? ...
... Note: specific heat of water is 4.18 joules/gramC (which means that it takes 4.18 joules to raise one gram of water 1 C) Ex: How much heat energy in joules is absorbed by 30 grams of water (H2O) when it is heated from 20C to 30C? ...
Specific Heat Capacity
... 3. A 4.5 g nugget of pure gold absorbed 276 J of heat. What was the final temperature of the gold if the initial temperature was 25°C. The specific heat of gold is 0.129 J/(g°C). ...
... 3. A 4.5 g nugget of pure gold absorbed 276 J of heat. What was the final temperature of the gold if the initial temperature was 25°C. The specific heat of gold is 0.129 J/(g°C). ...
heat
... Temperature change is important. Exact time is not important. Temperature will drift toward ambient before and after reaction Transition will be faster if NaOH is added rapidly and well stirred. (That is you will have a more nearly vertical temp. rise) ...
... Temperature change is important. Exact time is not important. Temperature will drift toward ambient before and after reaction Transition will be faster if NaOH is added rapidly and well stirred. (That is you will have a more nearly vertical temp. rise) ...
Thermal Expansion and Temperature Scales
... 4. How does energy from the sun travel to Earth? 5. The heat from a pot on the stove moves to the pot’s handle by __________. 6. Tanning lamps transfer thermal energy primarily by which of the above means? 7. As you sit across the room from a fireplace, you feel its warmth due to heat transferred by ...
... 4. How does energy from the sun travel to Earth? 5. The heat from a pot on the stove moves to the pot’s handle by __________. 6. Tanning lamps transfer thermal energy primarily by which of the above means? 7. As you sit across the room from a fireplace, you feel its warmth due to heat transferred by ...
File
... 2. What is the relationship between absolute zero and thermal energy? At absolute zero there is no thermal energy 3. Compare and contrast heat and temperature Heat is the energy transferred from a high temperature system to a low temperature system. The more heat, the higher the temp and vice versa. ...
... 2. What is the relationship between absolute zero and thermal energy? At absolute zero there is no thermal energy 3. Compare and contrast heat and temperature Heat is the energy transferred from a high temperature system to a low temperature system. The more heat, the higher the temp and vice versa. ...
40Animal Structure - Mid
... used by animals. – Birds and mammals are mainly endothermic, maintaining their body temperature at a certain level with heat generated by metabolism. • Endothermy is a high-energy strategy that permits intense, long-duration activity of a wide range of environmental temperatures. ...
... used by animals. – Birds and mammals are mainly endothermic, maintaining their body temperature at a certain level with heat generated by metabolism. • Endothermy is a high-energy strategy that permits intense, long-duration activity of a wide range of environmental temperatures. ...
... a) If a block of aluminum (p = 2700 kgim3, Cp= 0.903 kJ/kg. K) and a block of steel (p = 8930 kgim3, cp = 0.385 kJ/kg.K) having the same volume received the same amount of energy by heat transfer (oQ), which one would experience the greater temperature increase? b) What are some of the principal irr ...
Cooling, thermal resistance, modeling of heat transfer as an electric
... Internal circulation of air flow ...
... Internal circulation of air flow ...
specific heat of water = 4.18 J/g•°C heat of vaporization of water
... 6) An underwater explosion caused the temperature of a pond to change from 76.0oC to 78.5oC. If the pond has a volume of 10,500 L, how many kilojoules of heat was released by the explosion? What law allows one to assume all heat lost from the explosion was absorbed by the water? Hint: D water = 1.0 ...
... 6) An underwater explosion caused the temperature of a pond to change from 76.0oC to 78.5oC. If the pond has a volume of 10,500 L, how many kilojoules of heat was released by the explosion? What law allows one to assume all heat lost from the explosion was absorbed by the water? Hint: D water = 1.0 ...
Lecture 14a – Introduction to Animal Function
... 3. Circulatory systems transport materials by bulk flow between exchange surfaces and body cells. - exchange by diffusion is only effective over very short distances - larger vessels (arteries and veins) carry blood over longer distances; smallest vessels (capillaries) function for exchange; located ...
... 3. Circulatory systems transport materials by bulk flow between exchange surfaces and body cells. - exchange by diffusion is only effective over very short distances - larger vessels (arteries and veins) carry blood over longer distances; smallest vessels (capillaries) function for exchange; located ...
Course 2 – Mathematical Tools and Unit Conversion Used in
... Gas constant R Ideal gas obeys Boyle’s law, Charle’s law and Avogadro’s law ...
... Gas constant R Ideal gas obeys Boyle’s law, Charle’s law and Avogadro’s law ...
General Chemistry: Chemistry 1000
... B. Temperature and energy determinations Put a known volume of room temperature water in your Erlenmeyer (don’t fill it all the way up). a. Measure the temperature of the water. T1 = __________ + ___________. b. Heat the water with a hot plate until the water boils. Measure the temperature of the bo ...
... B. Temperature and energy determinations Put a known volume of room temperature water in your Erlenmeyer (don’t fill it all the way up). a. Measure the temperature of the water. T1 = __________ + ___________. b. Heat the water with a hot plate until the water boils. Measure the temperature of the bo ...
Matt Johnson - Humboldt State University
... A. Birds are endothermic, maintaining a high body temperature around 40 C (higher than most mammals). This high temperature allows high levels of sustained activity, even when ambient temperatures are low; birds live in the earth's hottest deserts to its coldest seas. B. To maintain this high tempe ...
... A. Birds are endothermic, maintaining a high body temperature around 40 C (higher than most mammals). This high temperature allows high levels of sustained activity, even when ambient temperatures are low; birds live in the earth's hottest deserts to its coldest seas. B. To maintain this high tempe ...
Hyperthermia
Hyperthermia is elevated body temperature due to failed thermoregulation that occurs when a body produces or absorbs more heat than it dissipates. Extreme temperature elevation then becomes a medical emergency requiring immediate treatment to prevent disability or death.The most common causes include heat stroke and adverse reactions to drugs. The former is an acute temperature elevation caused by exposure to excessive heat, or combination of heat and humidity, that overwhelms the heat-regulating mechanisms. The latter is a relatively rare side effect of many drugs, particularly those that affect the central nervous system. Malignant hyperthermia is a rare complication of some types of general anesthesia.Hyperthermia differs from fever in that the body's temperature set point remains unchanged. The opposite is hypothermia, which occurs when the temperature drops below that required to maintain normal metabolism.























