PFC_MOF_McKellar_crsytengcomm_refereeresponses
... quantity of gas that can be stored in the material. Evaluation of gas storage capacities is usually performed using gravimetric or calorimetric analysis over a range of temperatures and pressures. However, in the alternative method outlined here, we have used high-pressure, single-crystal X-ray diff ...
... quantity of gas that can be stored in the material. Evaluation of gas storage capacities is usually performed using gravimetric or calorimetric analysis over a range of temperatures and pressures. However, in the alternative method outlined here, we have used high-pressure, single-crystal X-ray diff ...
3. Experimental apparatus
... HPC-5000. Fig. 3.6. shows the temperature measurement system used in the present work. The air bath sample room was a 70-mm diameter steel cylinder with a height of 130 mm. The apparatus was specially designed in the present work. As the sample was explosive material, the amount of the sample should ...
... HPC-5000. Fig. 3.6. shows the temperature measurement system used in the present work. The air bath sample room was a 70-mm diameter steel cylinder with a height of 130 mm. The apparatus was specially designed in the present work. As the sample was explosive material, the amount of the sample should ...
AL COS #
... This is not the test. This is a guide to the type of question that will appear on the test. ...
... This is not the test. This is a guide to the type of question that will appear on the test. ...
REGENTS Review Homework
... Cools fast (extrusive): small/no/fine crystals -air pockets (pumice) Cools slow (intrusive): large (coarse) crystals -ex: granite MINERALS they contain are below ...
... Cools fast (extrusive): small/no/fine crystals -air pockets (pumice) Cools slow (intrusive): large (coarse) crystals -ex: granite MINERALS they contain are below ...
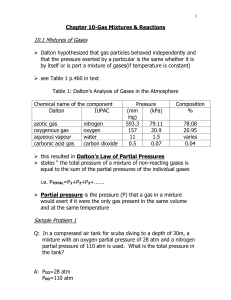
12.1 Avogadro`s Law and Molar Volume
... molecules as a container with 1.0 L of N2 at STP, even though the mass of nitrogen is 14 times greater. The pressure is directly related to the number of particles in each container of gas. So if each container had the same amount of pressure, there would be no difference in the number of particles. ...
... molecules as a container with 1.0 L of N2 at STP, even though the mass of nitrogen is 14 times greater. The pressure is directly related to the number of particles in each container of gas. So if each container had the same amount of pressure, there would be no difference in the number of particles. ...
Test 1
... 5. Define the following terms (2 points each) Heat of formation - Energy gained or lost when a compound in its standard state if forms from its elements in their standard state. Molar Bond energy - The energy required to break 1 mole of a given type of chemical bond. Hess’s Law - If you add two or ...
... 5. Define the following terms (2 points each) Heat of formation - Energy gained or lost when a compound in its standard state if forms from its elements in their standard state. Molar Bond energy - The energy required to break 1 mole of a given type of chemical bond. Hess’s Law - If you add two or ...
Chemistry I
... 28. For a gas with temperature and number of moles are held constant, Boyle’s law describes a situation in which: a. volume and pressure have no relationship b. volume increases with increasing pressure c. volume decreases with decreasing speed d. volume decreases with increasing pressure ...
... 28. For a gas with temperature and number of moles are held constant, Boyle’s law describes a situation in which: a. volume and pressure have no relationship b. volume increases with increasing pressure c. volume decreases with decreasing speed d. volume decreases with increasing pressure ...
Atomic spectroscopy methods
... light absorption and emission of atoms in the gas phase. The goal is elemental analysis - identity and concentration • of a specific element in the sample; chemical and structural information are lost. The sample is destroyed. ...
... light absorption and emission of atoms in the gas phase. The goal is elemental analysis - identity and concentration • of a specific element in the sample; chemical and structural information are lost. The sample is destroyed. ...
Honors Chemistry Final Review
... When given the formula, and writing the name for ionic compounds, remember to place the names in the same order as they appear in the formula. Remember, that if a transition metal, tin or lead appears, you will need a Roman Numeral. The Roman Numeral indicates the charge of the metal ion, and while ...
... When given the formula, and writing the name for ionic compounds, remember to place the names in the same order as they appear in the formula. Remember, that if a transition metal, tin or lead appears, you will need a Roman Numeral. The Roman Numeral indicates the charge of the metal ion, and while ...
2014-15 FINAL REVIEW Nomenclature: Chemical Name Chemical
... (a) If 37.5 grams of Fe2O3 is produced, find the moles of Fe that react. (b) If 37.5 grams of Fe2O3 is produced, find the volume of O2 that reacts at STP. (c) If 37.5 grams of Fe2O3 is produced, find the volume of O2 that reacts at 1.1 atm and 25°C. (d) What mass of Fe is needed to react with 13.0 L ...
... (a) If 37.5 grams of Fe2O3 is produced, find the moles of Fe that react. (b) If 37.5 grams of Fe2O3 is produced, find the volume of O2 that reacts at STP. (c) If 37.5 grams of Fe2O3 is produced, find the volume of O2 that reacts at 1.1 atm and 25°C. (d) What mass of Fe is needed to react with 13.0 L ...
Gas Laws Powerpoint
... There are several laws we use to quantify the behavior of gases The laws describe some combination of changes in pressure (P), volume (V), moles/amount of gas (n),or temperature (T) Standard Temperature and Pressure (STP) ◦ 0ºC and 1 atm ◦ 1 mole of gas occupies 22.4 L ...
... There are several laws we use to quantify the behavior of gases The laws describe some combination of changes in pressure (P), volume (V), moles/amount of gas (n),or temperature (T) Standard Temperature and Pressure (STP) ◦ 0ºC and 1 atm ◦ 1 mole of gas occupies 22.4 L ...
Second Semester Extra Review
... 6. A mixture of gases is at a pressure of 920.3 mmHg, what is the partial pressure of carbon dioxide gas in the mixture containing oxygen and carbon dioxide if the partial pressure of oxygen is 524.9 mmHg? 7. What is standard temperature and pressure? 8. An oxygen gas cylinder has a volume of 5.6 x ...
... 6. A mixture of gases is at a pressure of 920.3 mmHg, what is the partial pressure of carbon dioxide gas in the mixture containing oxygen and carbon dioxide if the partial pressure of oxygen is 524.9 mmHg? 7. What is standard temperature and pressure? 8. An oxygen gas cylinder has a volume of 5.6 x ...
C:\Users\Jim\Documents\usb key backups\Nov. 17\sch3u\unit 4
... Note just how fast air molecules move on average, even at room temperature (300 K): 500 m/s or 0.5 km/s. Higher temperatures mean higher average speed. However, temperature is proportional to kinetic energy, not speed. Ek = .5 m v2 At the same temperature, molecules with larger molar masses are movi ...
... Note just how fast air molecules move on average, even at room temperature (300 K): 500 m/s or 0.5 km/s. Higher temperatures mean higher average speed. However, temperature is proportional to kinetic energy, not speed. Ek = .5 m v2 At the same temperature, molecules with larger molar masses are movi ...
117 Ways to Pass the Earth Science Regents
... 1. The same substance always has the same density ...
... 1. The same substance always has the same density ...
a Gas
... - has neither a shape of its own nor fixed volume. It takes the shape and volume of its container. - Gas mixtures are always homogeneous - Gases are highly compressible. - The molecules of a gas are relatively far away each other. - Individual gas molecules have little interaction with their neighbo ...
... - has neither a shape of its own nor fixed volume. It takes the shape and volume of its container. - Gas mixtures are always homogeneous - Gases are highly compressible. - The molecules of a gas are relatively far away each other. - Individual gas molecules have little interaction with their neighbo ...
CHEM 150
... ____ 26. Which of the following affects the vapor pressure of a liquid? a. the mass of the sample b. the shape the sample c. the temperature of the sample d. the volume of the sample ____ 27. Chloroform has a normal boiling point of 61.7oC. Which of the following is true? a. at any temperature the ...
... ____ 26. Which of the following affects the vapor pressure of a liquid? a. the mass of the sample b. the shape the sample c. the temperature of the sample d. the volume of the sample ____ 27. Chloroform has a normal boiling point of 61.7oC. Which of the following is true? a. at any temperature the ...
1411-practice exam 2(ch4 5) - Chemistry
... Only available on studygur Topic: Download(s): 0 Pages: 4 Published: 26 октября 2016 READ FULL DOCUMENT Please sign up to read full document. TEXT PREVIEW ...
... Only available on studygur Topic: Download(s): 0 Pages: 4 Published: 26 октября 2016 READ FULL DOCUMENT Please sign up to read full document. TEXT PREVIEW ...
A Study of YVO4 - Sweet Briar College
... replace the popular YAG crystal in diode pumped lasers used in scientific, medical and military applications. When compared to the YAG crystal, YVO4 is found to be potentially more efficient. However when YVO4 is grown, defects are often observed in the boule. For our research we set out to study th ...
... replace the popular YAG crystal in diode pumped lasers used in scientific, medical and military applications. When compared to the YAG crystal, YVO4 is found to be potentially more efficient. However when YVO4 is grown, defects are often observed in the boule. For our research we set out to study th ...
42.89 KB
... 16. The pressure of ozone (O3) in the atmosphere is 1.4 x 10-7 atm and the temperature is -23oC. How many moles of ozone are in 1.0 L under these conditions? A) B) C) D) E) ...
... 16. The pressure of ozone (O3) in the atmosphere is 1.4 x 10-7 atm and the temperature is -23oC. How many moles of ozone are in 1.0 L under these conditions? A) B) C) D) E) ...
practice test2
... A) The temperature of steam cannot exceed 100°C. B) The temperature of ice remains at 0°C as it melts. C) The temperature of liquid water increases linearly as it is heated D) The temperature of liquid water remains at 100°C as it boils E) Both liquid water and ice are present at 0°C. ...
... A) The temperature of steam cannot exceed 100°C. B) The temperature of ice remains at 0°C as it melts. C) The temperature of liquid water increases linearly as it is heated D) The temperature of liquid water remains at 100°C as it boils E) Both liquid water and ice are present at 0°C. ...
dfafafad
... Sinking air creates: HIGH PRESSURE If air is sinking, then air is pushing down on the surface of earth with a amount of pressure. ...
... Sinking air creates: HIGH PRESSURE If air is sinking, then air is pushing down on the surface of earth with a amount of pressure. ...
Chapter 14…Kinetic Theory
... 7. Indicate the relationship between the variables in each of the equations above. 8. Indicate what each variable stands for in each of the equations above. 9. Temperature is in what unit for gas laws? 10. STP stands for: 11. Standard temperature = __________ 12. Standard pressure = __________ atm, ...
... 7. Indicate the relationship between the variables in each of the equations above. 8. Indicate what each variable stands for in each of the equations above. 9. Temperature is in what unit for gas laws? 10. STP stands for: 11. Standard temperature = __________ 12. Standard pressure = __________ atm, ...
Synthesis of single crystal diamond and its applications
... towards modifying CVD reactor geometry, and towards achieving uniform plasma power density distribution across the surface of diamond substrate. Nearly colorless and colorless diamonds have been produced as a result. Diamonds that have a slight hue have been annealed in order to obtain colorless dia ...
... towards modifying CVD reactor geometry, and towards achieving uniform plasma power density distribution across the surface of diamond substrate. Nearly colorless and colorless diamonds have been produced as a result. Diamonds that have a slight hue have been annealed in order to obtain colorless dia ...
Diamond anvil cell
A diamond anvil cell (DAC) is a device used in scientific experiments. It allows compressing a small (sub-millimeter-sized) piece of material to extreme pressures, which can exceed 600 gigapascals (6,000,000 bars / 6 million atmospheres).The device has been used to recreate the pressure existing deep inside planets, creating materials and phases not observed under normal conditions. Notable examples include the non-molecular ice X, polymeric nitrogen and metallic xenon (an inert gas at lower pressures).A DAC consists of two opposing diamonds with a sample compressed between the culets (tips). Pressure may be monitored using a reference material whose behavior under pressure is known. Common pressure standards include ruby fluorescence, and various structurally simple metals, such as copper or platinum. The uniaxial pressure supplied by the DAC may be transformed into uniform hydrostatic pressure using a pressure transmitting medium, such as argon, xenon, hydrogen, helium, paraffin oil or a mixture of methanol and ethanol. The pressure-transmitting medium is enclosed by a gasket and the two diamond anvils. The sample can be viewed through the diamonds and illuminated by X-rays and visible light. In this way, X-ray diffraction and fluorescence; optical absorption and photoluminescence; Mössbauer, Raman and Brillouin scattering; positron annihilation and other signals can be measured from materials under high pressure. Magnetic and microwave fields can be applied externally to the cell allowing nuclear magnetic resonance, electron paramagnetic resonance and other magnetic measurements. Attaching electrodes to the sample allows electrical and magnetoelectrical measurements as well as heating up the sample to a few thousand degrees. Much higher temperatures (up to 7000 K) can be achieved with laser-induced heating, and cooling down to millikelvins has been demonstrated.