Section 4.9 Oxidation–Reduction Reactions
... results from the combustion of fossil fuels. • This is the balanced equation for the combustion of octane (gasoline) • 2 C8H18 (l) + 25 O2 (g) → 16 CO2 (g) + 18 H2O (g) ...
... results from the combustion of fossil fuels. • This is the balanced equation for the combustion of octane (gasoline) • 2 C8H18 (l) + 25 O2 (g) → 16 CO2 (g) + 18 H2O (g) ...
Characteristics of Living Things
... 2. No, volcanoes are not living things. Although they appear to possess some of the characteristics of living things such as growth and breathing out waste gases, they do not possess all the characteristics. For example, volcanoes are not make of cells. 3. A. Plant is responds to environmental stimu ...
... 2. No, volcanoes are not living things. Although they appear to possess some of the characteristics of living things such as growth and breathing out waste gases, they do not possess all the characteristics. For example, volcanoes are not make of cells. 3. A. Plant is responds to environmental stimu ...
3-energy
... the absence of an enzyme catalyst. This kinetic stability is essential to the role of ATP and other compounds with ~ bonds. If ATP would rapidly hydrolyze in the absence of a catalyst, it could not serve its important roles in energy metabolism and phosphate transfer. Phosphate is removed from ATP o ...
... the absence of an enzyme catalyst. This kinetic stability is essential to the role of ATP and other compounds with ~ bonds. If ATP would rapidly hydrolyze in the absence of a catalyst, it could not serve its important roles in energy metabolism and phosphate transfer. Phosphate is removed from ATP o ...
The molecular machinery of Keilin`s respiratory chain
... FMN and has the NADH-binding site and also houses one of the Fe4 S4 centres, N3. It is associated with a 24 kDa subunit, likely to contain the Fe2 S2 centre N1b, and a 75 kDa subunit, which probably contains three or four iron– sulphur centres. These three subunits have homology with the NADH oxidor ...
... FMN and has the NADH-binding site and also houses one of the Fe4 S4 centres, N3. It is associated with a 24 kDa subunit, likely to contain the Fe2 S2 centre N1b, and a 75 kDa subunit, which probably contains three or four iron– sulphur centres. These three subunits have homology with the NADH oxidor ...
Cellular Energy and Mitochondrial ATP Production: A
... individual will experience. Optimal metabolism results in the 100 trillion human cells to function at peak performance. A person whose metabolism is functioning in such a manner will have an abundance of energy and a sharp mind to enjoy life and handle its many challenges with few worries of becomin ...
... individual will experience. Optimal metabolism results in the 100 trillion human cells to function at peak performance. A person whose metabolism is functioning in such a manner will have an abundance of energy and a sharp mind to enjoy life and handle its many challenges with few worries of becomin ...
Question Paper
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
MCAT 2015
... Phosphoryl group transfers and ATP o ATP hydrolysis ΔG << 0 o ATP group transfers Biological oxidation-‐reduction o Half-‐reactions o Soluble electron carriers o Flavoproteins ...
... Phosphoryl group transfers and ATP o ATP hydrolysis ΔG << 0 o ATP group transfers Biological oxidation-‐reduction o Half-‐reactions o Soluble electron carriers o Flavoproteins ...
Condensation is a chemical reaction in which one molecule is
... two amino acids to form a peptide. This reaction example is the reverse of hydrolysis, which splits a chemical entity into two parts through action from the polar water molecule, which itself splits into hydroxide and hydrogen ions. ...
... two amino acids to form a peptide. This reaction example is the reverse of hydrolysis, which splits a chemical entity into two parts through action from the polar water molecule, which itself splits into hydroxide and hydrogen ions. ...
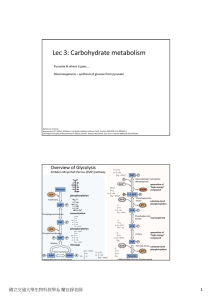
Lec 3: Carbohydrate metabolism
... use glucose as their sole or primary energy source, but they lack the enzymatic machinery to synthesize it. Liver and kidney cortex are the primary gluconeogenic tissues. ...
... use glucose as their sole or primary energy source, but they lack the enzymatic machinery to synthesize it. Liver and kidney cortex are the primary gluconeogenic tissues. ...
Word
... C) Galactokinase and galactose 1-phosphate uridyl transferase deficiencies cause mental retardation D) Galactose 1-phosphate uridyl transferase releases free glucose E) UDP-galactose requires a phosphoglucomutase to convert it to UDP-Glucose 21) Malonyl CoA, which was 14C labeled (a radioactive isot ...
... C) Galactokinase and galactose 1-phosphate uridyl transferase deficiencies cause mental retardation D) Galactose 1-phosphate uridyl transferase releases free glucose E) UDP-galactose requires a phosphoglucomutase to convert it to UDP-Glucose 21) Malonyl CoA, which was 14C labeled (a radioactive isot ...
Quatrom
... The earth’s two main sources of oxygen are from plankton in the world’s oceans and new growth in the rain forest of Africa, Central and South America. Recent studies indicate that our atmospheric oxygen levels have decreased significantly during the last century. In fact, in some of the larger indus ...
... The earth’s two main sources of oxygen are from plankton in the world’s oceans and new growth in the rain forest of Africa, Central and South America. Recent studies indicate that our atmospheric oxygen levels have decreased significantly during the last century. In fact, in some of the larger indus ...
Document
... into carbon skeletons. Ammonium can be formed from oxidized inorganic percursors by reductive reactions: nitrogen fixation reduces N2 to NH4+; nitrate assimilation reduces NO3- to NH4+. Nitrifying bacteria can oxidize NH4+ back to NO3- and obtain energy for growth in the process of nitrification. De ...
... into carbon skeletons. Ammonium can be formed from oxidized inorganic percursors by reductive reactions: nitrogen fixation reduces N2 to NH4+; nitrate assimilation reduces NO3- to NH4+. Nitrifying bacteria can oxidize NH4+ back to NO3- and obtain energy for growth in the process of nitrification. De ...
Plant Physiology Online: Gibberellin Biosynthesis
... give ent-kaurenoic acid (KA) (see Figure 3). These oxidations are catalyzed by ent-kaurene oxidase (KO), which is said to be multifunctional because it can catalyze all three reactions. In Arabidopsis, KO is encoded by GA3, and mutations in this gene will again produce severely dwarfed plants. Kaure ...
... give ent-kaurenoic acid (KA) (see Figure 3). These oxidations are catalyzed by ent-kaurene oxidase (KO), which is said to be multifunctional because it can catalyze all three reactions. In Arabidopsis, KO is encoded by GA3, and mutations in this gene will again produce severely dwarfed plants. Kaure ...
proposal jano
... of today’s medicines are either obtained directly from natural source or were developed from a lead compound originally obtained from a natural source (Graham 2001). In Kenya 75 plants species from 34 families are used to cure 59 ailments in traditional medicine of central Kenya, 80% of South Africa ...
... of today’s medicines are either obtained directly from natural source or were developed from a lead compound originally obtained from a natural source (Graham 2001). In Kenya 75 plants species from 34 families are used to cure 59 ailments in traditional medicine of central Kenya, 80% of South Africa ...
lecture1
... basicity is difficult, potential is widely positive. Those with strong basicity have narrowly positive potentials and are easily oxidized. Strong oxidizing agent is stable in weakly basic solvents and substances difficult to oxidize can be oxidized in them. But unstable in strongly basic solvents. ...
... basicity is difficult, potential is widely positive. Those with strong basicity have narrowly positive potentials and are easily oxidized. Strong oxidizing agent is stable in weakly basic solvents and substances difficult to oxidize can be oxidized in them. But unstable in strongly basic solvents. ...
Chapter 9 (Jan 27-29)
... Substrate-level phosphorylation – ATP produced from the transfer of a phosphate group from a substrate to ADP ATP made one at a time Enzyme ...
... Substrate-level phosphorylation – ATP produced from the transfer of a phosphate group from a substrate to ADP ATP made one at a time Enzyme ...
Enzymes
... formed. ●If reactants do not have enough energy, no reaction will take place. Copyright Pearson Prentice Hall ...
... formed. ●If reactants do not have enough energy, no reaction will take place. Copyright Pearson Prentice Hall ...
BCHM 463 Supplemental Problems for Friday, April 2, 2004 1. Write
... answer with regard to your answer to #1. 4 ADP molecules are converted into ATP. There is a net gain of only 2 ATP molecules because 2 are consumed during the first stage of glycolysis. 3. What are the three metabolically irreversible steps of glycolysis? What general type of reaction is catalyzed b ...
... answer with regard to your answer to #1. 4 ADP molecules are converted into ATP. There is a net gain of only 2 ATP molecules because 2 are consumed during the first stage of glycolysis. 3. What are the three metabolically irreversible steps of glycolysis? What general type of reaction is catalyzed b ...
Growth and pigmentation of marine infrared radiation (IR) absorbing
... light, IR, and dark experimental conditions. Enrichments with H2CO2 media resulted in colored growth under all light conditions, with greatest pigment production in light and IR treatments (Figure 4b). The light and IR treatments were found to contain major absorbance peaks at 860, 790, 590, and 450 ...
... light, IR, and dark experimental conditions. Enrichments with H2CO2 media resulted in colored growth under all light conditions, with greatest pigment production in light and IR treatments (Figure 4b). The light and IR treatments were found to contain major absorbance peaks at 860, 790, 590, and 450 ...
Sample Chapters - Pearson Canada
... with storing and generating metabolic energy and with using that energy in biosynthesis of low-molecular-weight compounds (intermediates) and energystorage compounds. Not included are nucleic acid and protein biosynthesis from monomeric precursors. The reactions of intermediary metabolism can be tho ...
... with storing and generating metabolic energy and with using that energy in biosynthesis of low-molecular-weight compounds (intermediates) and energystorage compounds. Not included are nucleic acid and protein biosynthesis from monomeric precursors. The reactions of intermediary metabolism can be tho ...
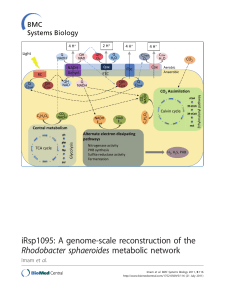
iRsp1095: A genome-scale reconstruction of the Rhodobacter
... Background: Rhodobacter sphaeroides is one of the best studied purple non-sulfur photosynthetic bacteria and serves as an excellent model for the study of photosynthesis and the metabolic capabilities of this and related facultative organisms. The ability of R. sphaeroides to produce hydrogen (H2), ...
... Background: Rhodobacter sphaeroides is one of the best studied purple non-sulfur photosynthetic bacteria and serves as an excellent model for the study of photosynthesis and the metabolic capabilities of this and related facultative organisms. The ability of R. sphaeroides to produce hydrogen (H2), ...
File
... a lower efficiency in energy production from glycolysis. • Complete oxidation of CO2 in healthy cells under aerobic conditions yields ~30 ATP per glucose. • Anaerobic metabolism of glucose in tumor cells yields 2 ATP per glucose. – Glucose transporters and most glycolytic enzymes are overexpressed i ...
... a lower efficiency in energy production from glycolysis. • Complete oxidation of CO2 in healthy cells under aerobic conditions yields ~30 ATP per glucose. • Anaerobic metabolism of glucose in tumor cells yields 2 ATP per glucose. – Glucose transporters and most glycolytic enzymes are overexpressed i ...
Photosynthesis

Photosynthesis is a process used by plants and other organisms to convert light energy, normally from the Sun, into chemical energy that can be later released to fuel the organisms' activities. This chemical energy is stored in carbohydrate molecules, such as sugars, which are synthesized from carbon dioxide and water – hence the name photosynthesis, from the Greek φῶς, phōs, ""light"", and σύνθεσις, synthesis, ""putting together"". In most cases, oxygen is also released as a waste product. Most plants, most algae, and cyanobacteria perform photosynthesis; such organisms are called photoautotrophs. Photosynthesis maintains atmospheric oxygen levels and supplies all of the organic compounds and most of the energy necessary for life on Earth.Although photosynthesis is performed differently by different species, the process always begins when energy from light is absorbed by proteins called reaction centres that contain green chlorophyll pigments. In plants, these proteins are held inside organelles called chloroplasts, which are most abundant in leaf cells, while in bacteria they are embedded in the plasma membrane. In these light-dependent reactions, some energy is used to strip electrons from suitable substances, such as water, producing oxygen gas. Furthermore, two further compounds are generated: reduced nicotinamide adenine dinucleotide phosphate (NADPH) and adenosine triphosphate (ATP), the ""energy currency"" of cells.In plants, algae and cyanobacteria, sugars are produced by a subsequent sequence of light-independent reactions called the Calvin cycle, but some bacteria use different mechanisms, such as the reverse Krebs cycle. In the Calvin cycle, atmospheric carbon dioxide is incorporated into already existing organic carbon compounds, such as ribulose bisphosphate (RuBP). Using the ATP and NADPH produced by the light-dependent reactions, the resulting compounds are then reduced and removed to form further carbohydrates, such as glucose.The first photosynthetic organisms probably evolved early in the evolutionary history of life and most likely used reducing agents, such as hydrogen or hydrogen sulfide, as sources of electrons, rather than water. Cyanobacteria appeared later; the excess oxygen they produced contributed to the oxygen catastrophe, which rendered the evolution of complex life possible. Today, the average rate of energy capture by photosynthesis globally is approximately 130 terawatts, which is about three times the current power consumption of human civilization.Photosynthetic organisms also convert around 100–115 thousand million metric tonnes of carbon into biomass per year.