Citric acid cycle
... • Before the citric acid cycle can begin – Pyruvate must first be converted to acetyl CoA, which links the cycle to glycolysis – Fully oxidized carboxyl group is removed as a CO2 – The remaining two-carbon fragment is oxidized to acetate. The extracted electrons are transferred to NAD+, forming NAD ...
... • Before the citric acid cycle can begin – Pyruvate must first be converted to acetyl CoA, which links the cycle to glycolysis – Fully oxidized carboxyl group is removed as a CO2 – The remaining two-carbon fragment is oxidized to acetate. The extracted electrons are transferred to NAD+, forming NAD ...
Citric Acid Cycle
... Electrons from these oxidation processes are then used to reduce oxygen to water with the concomitant formation of ATP. The unique structural aspects of mitochondria facilitate its energy-harvesting role. ...
... Electrons from these oxidation processes are then used to reduce oxygen to water with the concomitant formation of ATP. The unique structural aspects of mitochondria facilitate its energy-harvesting role. ...
Bioenergetics
... Fast glycolysis occurs during reduced oxygen availability and the end product is lactic acid. Lactic acid accumulation in tissue is the result of an imbalance of production & utilization. As lactic acid accumulates, there is an increase in the concentration of H++ ions. H++ ions inhibit glycolytic r ...
... Fast glycolysis occurs during reduced oxygen availability and the end product is lactic acid. Lactic acid accumulation in tissue is the result of an imbalance of production & utilization. As lactic acid accumulates, there is an increase in the concentration of H++ ions. H++ ions inhibit glycolytic r ...
Metabolic Fate of Glucose Metabolic Fate of Fatty Acids
... Type I and Type II Muscle Fibers • A short fast sprint is anaerobic exercise; a prolonged steady marathon is aerobic in nature. • ATP is required to power movement by muscle myosin. Muscle ATP stores are very limited. • There are two kinds of muscle fibers, type I and type II. • Type I fibers have e ...
... Type I and Type II Muscle Fibers • A short fast sprint is anaerobic exercise; a prolonged steady marathon is aerobic in nature. • ATP is required to power movement by muscle myosin. Muscle ATP stores are very limited. • There are two kinds of muscle fibers, type I and type II. • Type I fibers have e ...
The energy systems - TrackandFieldScience.com
... into the blood. This expulsion of these waste products prevents muscles from becoming quickly fatigued. With the ability of the heart and lungs to work harder to move greater volumes of oxygenated blood around the body the aerobic energy system allows the muscles to keep on working for extended peri ...
... into the blood. This expulsion of these waste products prevents muscles from becoming quickly fatigued. With the ability of the heart and lungs to work harder to move greater volumes of oxygenated blood around the body the aerobic energy system allows the muscles to keep on working for extended peri ...
Slide 1
... ETC As electrons pass down chain, H+ ions are pumped into inter-mitochondrial space making a charge gradient. Gradient provides energy for ATP synthase to add the P group. At the end of the chain, an enzyme combines the electrons with H+ and oxygen to form water, a by-product of electron ...
... ETC As electrons pass down chain, H+ ions are pumped into inter-mitochondrial space making a charge gradient. Gradient provides energy for ATP synthase to add the P group. At the end of the chain, an enzyme combines the electrons with H+ and oxygen to form water, a by-product of electron ...
09_Lecture_Presentation
... Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings ...
... Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings ...
RedOx notes:
... If the substance is ionic, are there any monoatomic ions present? Which elements have specific rules? Which element(s) do(es) not have rules? Use rule 8 or 9 from above to calculate these. ...
... If the substance is ionic, are there any monoatomic ions present? Which elements have specific rules? Which element(s) do(es) not have rules? Use rule 8 or 9 from above to calculate these. ...
PPT - Chris Anthony
... The dehydrogenase is usually a flavoprotein that interacts like other flavoproteins with the electron transport chain at the level of ubiquinone and cytochrome b. (There are some reports of NAD-linked dehydrogenases in some Pseudomonas and Hyphomicrobia Netrusov) ...
... The dehydrogenase is usually a flavoprotein that interacts like other flavoproteins with the electron transport chain at the level of ubiquinone and cytochrome b. (There are some reports of NAD-linked dehydrogenases in some Pseudomonas and Hyphomicrobia Netrusov) ...
Amino Acid Catabolism - Chemistry Courses: About
... • DHF must be reduced to THF by DHF reductase • NADPH dependent • Chemotherapy dtarget – DHF analogs such as methotrexate ...
... • DHF must be reduced to THF by DHF reductase • NADPH dependent • Chemotherapy dtarget – DHF analogs such as methotrexate ...
Amino Acid Catabolism - Chemistry Courses: About
... • DHF must be reduced to THF by DHF reductase • NADPH dependent • Chemotherapy dtarget – DHF analogs such as methotrexate ...
... • DHF must be reduced to THF by DHF reductase • NADPH dependent • Chemotherapy dtarget – DHF analogs such as methotrexate ...
... • Both are used to change the Gibbs free energy of a reaction from positive to negative to make that step in the pathway spontaneous (3 pts) • Direct coupling uses the energy released when ATP is converted to ADP + Pi to drive the reaction. The transfer of energy occurs within the active site of the ...
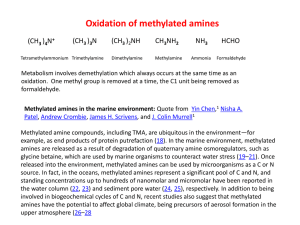
carbon compounds - Badhan Education
... Tetrahalogen Derivatives. They have general formula CnH2n – 2X4, e. g., CCI4 (carbon tetra-chloride). Denatured Spirit. Ethyl alcohol (ethanol) is mixed with poisonous substances like methyl alcohol (methanol), CuSO4, acetone and pyridine (C5H5N) so as to make it unfit for drinking and it is used as ...
... Tetrahalogen Derivatives. They have general formula CnH2n – 2X4, e. g., CCI4 (carbon tetra-chloride). Denatured Spirit. Ethyl alcohol (ethanol) is mixed with poisonous substances like methyl alcohol (methanol), CuSO4, acetone and pyridine (C5H5N) so as to make it unfit for drinking and it is used as ...
Slide 1
... to create a proton gradient across the membrane of the mitochondria – this potential energy provides the energy to convert ADP to ATP. 90% of ATP synthesis happens during electron transport. The “mobile” part of the ETS is a lipid-soluble compound called Coenzyme Q10 ...
... to create a proton gradient across the membrane of the mitochondria – this potential energy provides the energy to convert ADP to ATP. 90% of ATP synthesis happens during electron transport. The “mobile” part of the ETS is a lipid-soluble compound called Coenzyme Q10 ...
Test 1
... 4. Which one of the following types of mechanisms is not known to play a role in the reversible alteration of enzyme activity? A) Activation by cleavage of an inactive zymogen B) Allosteric response to a regulatory molecule C) Alteration of the synthesis or degradation rate of an enzyme D) Covalent ...
... 4. Which one of the following types of mechanisms is not known to play a role in the reversible alteration of enzyme activity? A) Activation by cleavage of an inactive zymogen B) Allosteric response to a regulatory molecule C) Alteration of the synthesis or degradation rate of an enzyme D) Covalent ...
Chapter 1
... • Acetyl CoA carries acetyl groups, 2carbon remnants of the nutrients • Acetyl CoA enters the citric acid cycle – Electrons and hydrogen atoms are harvested – Acetyl group is oxidized to produce CO2 – Electrons and hydrogen atoms harvested are used to produce ATP during oxidative ...
... • Acetyl CoA carries acetyl groups, 2carbon remnants of the nutrients • Acetyl CoA enters the citric acid cycle – Electrons and hydrogen atoms are harvested – Acetyl group is oxidized to produce CO2 – Electrons and hydrogen atoms harvested are used to produce ATP during oxidative ...
Regeneration of NAD+ Lactic Acid Fermentation
... • Mechanism involves two covalent intermediates with the enzyme: • Addition of pyruvate to TPP and loss of CO2 forms hydroxyethyl TPP. • (This same intermediate is formed by pyruvate decarboxylase in yeast alcoholic fermentation). ...
... • Mechanism involves two covalent intermediates with the enzyme: • Addition of pyruvate to TPP and loss of CO2 forms hydroxyethyl TPP. • (This same intermediate is formed by pyruvate decarboxylase in yeast alcoholic fermentation). ...
Airgas template
... – Energy is released. Catabolic reactions are a cell’s major source of energy. • Anabolic reactions involve the assembly of smaller molecules into larger molecules, requiring the formation of bonds. The bonds are stored energy. • Much of the energy released during catabolic reactions is used to buil ...
... – Energy is released. Catabolic reactions are a cell’s major source of energy. • Anabolic reactions involve the assembly of smaller molecules into larger molecules, requiring the formation of bonds. The bonds are stored energy. • Much of the energy released during catabolic reactions is used to buil ...
Food Biotechnology Dr. Tarek Elbashiti 7. Metabolic Engineering of
... • From reasoning based on metabolic pathways structure, rerouting a carbon source to produce a desired amino acid should start by increasing the availability of precursor metabolites, energy, and reducing equivalents used in its synthesis. • Central metabolic pathways meet these criteria, and theref ...
... • From reasoning based on metabolic pathways structure, rerouting a carbon source to produce a desired amino acid should start by increasing the availability of precursor metabolites, energy, and reducing equivalents used in its synthesis. • Central metabolic pathways meet these criteria, and theref ...
Ch 17- Carboxylic Acids and their derivatives
... – 1) The reaction can proceed through a six membered cyclic transition state to form an enol which tautomerises to the ketone – 2) When the carboxylate anion decarboxylates, it forms a resonance-stabilized enolate anion ...
... – 1) The reaction can proceed through a six membered cyclic transition state to form an enol which tautomerises to the ketone – 2) When the carboxylate anion decarboxylates, it forms a resonance-stabilized enolate anion ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)