C454_lect1 - University of Wisconsin
... Under standard conditions, the free energy for the hydrolysis of L-glycerol phosphate to form glycerol and inorganic phosphate is -2.2 kcal/mol. Calculate the factor by which the equilibrium ratio for the concentration of Lglycerol phosphate to glycerol is increased when ATP is used as the phosphory ...
... Under standard conditions, the free energy for the hydrolysis of L-glycerol phosphate to form glycerol and inorganic phosphate is -2.2 kcal/mol. Calculate the factor by which the equilibrium ratio for the concentration of Lglycerol phosphate to glycerol is increased when ATP is used as the phosphory ...
Name
... KEY CONCEPT Fermentation allows the production of a small amount of ATP without oxygen. When oxygen is not available in cells, fermentation takes place instead. Fermentation is an anaerobic process that allows glycolysis to continue, but does not produce ATP on its own. The main function of fermenta ...
... KEY CONCEPT Fermentation allows the production of a small amount of ATP without oxygen. When oxygen is not available in cells, fermentation takes place instead. Fermentation is an anaerobic process that allows glycolysis to continue, but does not produce ATP on its own. The main function of fermenta ...
Part I. Aim # 48- Levels of Interaction within an
... Deer ticks are responsible for spreading Lyme disease. This organism, which feeds on the blood of warm-blooded organisms like mice, deer, and humans, is best ...
... Deer ticks are responsible for spreading Lyme disease. This organism, which feeds on the blood of warm-blooded organisms like mice, deer, and humans, is best ...
Slide 1
... Pyruvate is oxidized prior to the citric acid cycle Two molecules of pyruvate are produced for each molecule of glucose that enters glycolysis. Pyruvate does not enter the citric acid cycle, but undergoes some chemical grooming in which – a carboxyl group is removed and given off as _______, – ...
... Pyruvate is oxidized prior to the citric acid cycle Two molecules of pyruvate are produced for each molecule of glucose that enters glycolysis. Pyruvate does not enter the citric acid cycle, but undergoes some chemical grooming in which – a carboxyl group is removed and given off as _______, – ...
pblock - Chemistry Courses
... 2nd period: Only s and p orbitals are possible with n = 2 Therefore, the maximum number of bonds is 4 (single and/or double bonds) Examples: CH4, NF4+, BH43rd (and higher periods): can use d-orbitals to make bonds E.g. ...
... 2nd period: Only s and p orbitals are possible with n = 2 Therefore, the maximum number of bonds is 4 (single and/or double bonds) Examples: CH4, NF4+, BH43rd (and higher periods): can use d-orbitals to make bonds E.g. ...
Cellular Respiration
... • We’ll only examine the most common fuel = sugar (C6H12O6) • Exergonic rxn: ∆G = -686 kcal/mol of Glucose (the energy will be used to generate ATP) ...
... • We’ll only examine the most common fuel = sugar (C6H12O6) • Exergonic rxn: ∆G = -686 kcal/mol of Glucose (the energy will be used to generate ATP) ...
How likely is it that life exists off the Earth?
... from signatures/models of planetary bodies that might have life or had life in the past? – Are there a subset of essential planetary conditions that can be measured remotely from exoplanets? ...
... from signatures/models of planetary bodies that might have life or had life in the past? – Are there a subset of essential planetary conditions that can be measured remotely from exoplanets? ...
Organic Chemistry DEFINE the following Vocabulary: Adhesion
... Carbohydrates and proteins are two types of macromolecules. Which functional characteristic of proteins distinguishes them from carbohydrates? A. large amount of stored information B. ability to catalyze biochemical reactions C. efficient storage of usable chemical energy D. tendency to make cell me ...
... Carbohydrates and proteins are two types of macromolecules. Which functional characteristic of proteins distinguishes them from carbohydrates? A. large amount of stored information B. ability to catalyze biochemical reactions C. efficient storage of usable chemical energy D. tendency to make cell me ...
Ecological Succession
... carbon, and nitrogen cycles) affect each other in any way? Explain. Each cycle is connected in many ways. Some forms of ______________________________________________________ nitrogen & carbon are carried through the environment by ______________________________________________________ water. Many n ...
... carbon, and nitrogen cycles) affect each other in any way? Explain. Each cycle is connected in many ways. Some forms of ______________________________________________________ nitrogen & carbon are carried through the environment by ______________________________________________________ water. Many n ...
Carbon Compounds
... The Chemistry of Carbon Carbon atoms have four valence electrons, allowing them to form strong covalent bonds with many other elements, including hydrogen, oxygen, phosphorus, sulfur, and nitrogen. Living organisms are made up of molecules that consist of carbon and these other elements. ...
... The Chemistry of Carbon Carbon atoms have four valence electrons, allowing them to form strong covalent bonds with many other elements, including hydrogen, oxygen, phosphorus, sulfur, and nitrogen. Living organisms are made up of molecules that consist of carbon and these other elements. ...
Adaptation by Natural Selection
... by the amount of water available. A few highly adapted desert organisms are able to store water for long periods of time. For example, cacti can store water in their large stems; however, most organisms that live on land must stay close to water sources and travel if a water source they use dries-up ...
... by the amount of water available. A few highly adapted desert organisms are able to store water for long periods of time. For example, cacti can store water in their large stems; however, most organisms that live on land must stay close to water sources and travel if a water source they use dries-up ...
Connections of Carbohydrate, Protein, and Lipid
... presence of glycogen as a source of glucose allows ATP to be produced for a longer period of time during exercise. Glycogen is broken down into G-1-P and converted into G-6-P in both muscle and liver cells, and this product enters the glycolytic pathway. Sucrose is a disaccharide with a molecule of ...
... presence of glycogen as a source of glucose allows ATP to be produced for a longer period of time during exercise. Glycogen is broken down into G-1-P and converted into G-6-P in both muscle and liver cells, and this product enters the glycolytic pathway. Sucrose is a disaccharide with a molecule of ...
Describe how cells are used in the production of
... (maximum of two) • Respiration is the process by which cells obtain/release/give out energy. • Respiration involves a series of enzyme-controlled reactions. • Aerobic respiration requires oxygen/takes place when oxygen is available. (maximum of two) • In the first stage glucose is broken down into p ...
... (maximum of two) • Respiration is the process by which cells obtain/release/give out energy. • Respiration involves a series of enzyme-controlled reactions. • Aerobic respiration requires oxygen/takes place when oxygen is available. (maximum of two) • In the first stage glucose is broken down into p ...
You Light Up My Life - Hawaii Community College
... hydrogen from intermediates during the first two stages When reduced, they are NADH and FADH2 In the third stage, these coenzymes deliver the electrons and hydrogen to the transfer chain ...
... hydrogen from intermediates during the first two stages When reduced, they are NADH and FADH2 In the third stage, these coenzymes deliver the electrons and hydrogen to the transfer chain ...
The Chemistry of Life
... • Acidity = measure of hydronium ions (H3O+) in a solution – Use pH scale to measure • pH 7 = neutral (pure water) • pH below 7 = acidic strongest is closer to 1 ...
... • Acidity = measure of hydronium ions (H3O+) in a solution – Use pH scale to measure • pH 7 = neutral (pure water) • pH below 7 = acidic strongest is closer to 1 ...
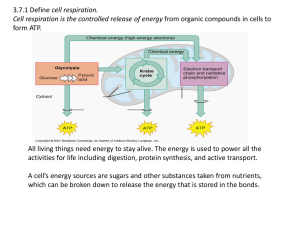
3.7:Cell Respiration Aerobic cell respiration: glucose
... cell respiration produces energy; controlled release of energy; by breakdown of organic molecules/glucose; energy from them is used to make ATP; aerobic respiration is in mitochondria; requires oxygen; pyruvate is produced by glycolysis / glucose broken down; pyruvate is broken down in the mitochond ...
... cell respiration produces energy; controlled release of energy; by breakdown of organic molecules/glucose; energy from them is used to make ATP; aerobic respiration is in mitochondria; requires oxygen; pyruvate is produced by glycolysis / glucose broken down; pyruvate is broken down in the mitochond ...
glycolysis and respiration
... inhibition byy high g energy end-products (ATP and NADH) and activation by indicators of low energy levels (ADP). I hibiti andd Inhibition activation both occur at steps early in glycolysis or respiration. Energy is harvested only when needed. ...
... inhibition byy high g energy end-products (ATP and NADH) and activation by indicators of low energy levels (ADP). I hibiti andd Inhibition activation both occur at steps early in glycolysis or respiration. Energy is harvested only when needed. ...
GLUCOSE HOMEOSTASIS – I: Brief Review of: AEROBIC
... Energy metabolism with emphasis on Glycolysis What is Glycolysis? • Glycolysis is a: • Major metabolic pathway for Energy production via degradation of Glucose and other Monosaccharides; • Unique pathway because it can occur: • In the presence of O2 (Aerobic Glycolysis) in cells that contain mitoch ...
... Energy metabolism with emphasis on Glycolysis What is Glycolysis? • Glycolysis is a: • Major metabolic pathway for Energy production via degradation of Glucose and other Monosaccharides; • Unique pathway because it can occur: • In the presence of O2 (Aerobic Glycolysis) in cells that contain mitoch ...
study guide
... 7. All heterotrophs must eat food to get energy. 8. Autotrophs do not need to eat food because they make food. 9. The energy in food originally came from ATP. 10. The term photosynthesis means “pulling apart with light” in Greek. 11. The energy of sunlight is stored in the chemical bonds of carbohyd ...
... 7. All heterotrophs must eat food to get energy. 8. Autotrophs do not need to eat food because they make food. 9. The energy in food originally came from ATP. 10. The term photosynthesis means “pulling apart with light” in Greek. 11. The energy of sunlight is stored in the chemical bonds of carbohyd ...
Document
... one cycle. Although the two carbons which enter the cycle become the part of oxaloacetate, and are released as CO2 only in the third round of the cycle. The energy released due to this oxidation is conserved in the reduction of 3 NAD+, 1 FAD molecule and synthesis of one GTP molecule which is conver ...
... one cycle. Although the two carbons which enter the cycle become the part of oxaloacetate, and are released as CO2 only in the third round of the cycle. The energy released due to this oxidation is conserved in the reduction of 3 NAD+, 1 FAD molecule and synthesis of one GTP molecule which is conver ...
Cellular Respiration 2016
... • Glycolysis generates 2 ATP whether oxygen is present (aerobic) or not (anaerobic). • Fermentation can generate ATP from glucose as long as there is a supply of NAD+ to accept electrons. • If the NAD+ pool is exhausted, glycolysis shuts down. ...
... • Glycolysis generates 2 ATP whether oxygen is present (aerobic) or not (anaerobic). • Fermentation can generate ATP from glucose as long as there is a supply of NAD+ to accept electrons. • If the NAD+ pool is exhausted, glycolysis shuts down. ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)