Chapter 3 Notes
... - chlorophyll is contained within the chloroplast The Light Reaction: see Figure 3.11 p. 89 Light energy enters the chloroplast (as it is translucent) and is absorbed by the chlorophyll contained within the thylakoid. The light causes water to break apart releasing oxygen (waste) and hydrogens which ...
... - chlorophyll is contained within the chloroplast The Light Reaction: see Figure 3.11 p. 89 Light energy enters the chloroplast (as it is translucent) and is absorbed by the chlorophyll contained within the thylakoid. The light causes water to break apart releasing oxygen (waste) and hydrogens which ...
How many molecules of adenosine triphosphate (ATP) can be
... List molecules, coenzymes, and ions that serve as electron carriers in cellular oxidation-reduction reactions. Name enzymes that use these molecules, coenzymes, and ions in catalysis. ...
... List molecules, coenzymes, and ions that serve as electron carriers in cellular oxidation-reduction reactions. Name enzymes that use these molecules, coenzymes, and ions in catalysis. ...
Metabolism
... 4 Types of Nitrogen Compounds 1. Amino acids: – framework of all proteins, glycoproteins, and lipoproteins ...
... 4 Types of Nitrogen Compounds 1. Amino acids: – framework of all proteins, glycoproteins, and lipoproteins ...
Biochemistry - El Camino College
... cells, digested to __________ in our bodies 3. ___________ - main component of plant cell walls; also known as _________; we can’t digest this because the covalent bonds between the glucose molecule are slightly different than those in starch and glycogen 4. Most carbohydrates are broken down to ___ ...
... cells, digested to __________ in our bodies 3. ___________ - main component of plant cell walls; also known as _________; we can’t digest this because the covalent bonds between the glucose molecule are slightly different than those in starch and glycogen 4. Most carbohydrates are broken down to ___ ...
AP Bio Test Questions
... mainly oxygen with carbon and hydrogen (hydrocarbons) hydrogen, oxygen, carbon, occasionally sulfur ...
... mainly oxygen with carbon and hydrogen (hydrocarbons) hydrogen, oxygen, carbon, occasionally sulfur ...
Characteristics of Living Things
... different functions (such as muscle cells for movement, blood cells to deliver O2) ...
... different functions (such as muscle cells for movement, blood cells to deliver O2) ...
Ch. 3 Study Guide
... 9. Name two VITALLY IMPORTANT monosaccharides A. B. 10. Monosaccharides, especially ______________, are the source of ___________ for cellular work. In addition, the carbon skeletons of monosaccharides provide the _____________________ for building other organic molecules like amino acids and fatty ...
... 9. Name two VITALLY IMPORTANT monosaccharides A. B. 10. Monosaccharides, especially ______________, are the source of ___________ for cellular work. In addition, the carbon skeletons of monosaccharides provide the _____________________ for building other organic molecules like amino acids and fatty ...
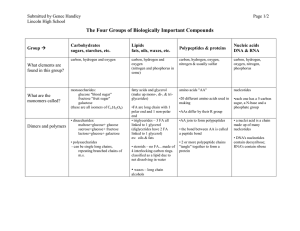
Macromolecules of Life Macromolecules of Life
... of carbohydrate, 4 kg of minerals and 40 kg of water, but the weight of nucleic acids in an organism is much less than the corresponding weights of other macromolecules Macromolecules of different classes interact with each other by forming covalent bonds and weaker bonds Proteins bind to carbohydra ...
... of carbohydrate, 4 kg of minerals and 40 kg of water, but the weight of nucleic acids in an organism is much less than the corresponding weights of other macromolecules Macromolecules of different classes interact with each other by forming covalent bonds and weaker bonds Proteins bind to carbohydra ...
BIO00004C Molecular biology and biochemistry (PDF , 72kb)
... introduction to lipid and carbohydrate structures, the role of the various macromolecules in the context of membrane flow, cell shape, etc. will be discussed. Energy and metabolism is introduced by discussing the important concept of free energy and relating this to the central role of ATP and coupl ...
... introduction to lipid and carbohydrate structures, the role of the various macromolecules in the context of membrane flow, cell shape, etc. will be discussed. Energy and metabolism is introduced by discussing the important concept of free energy and relating this to the central role of ATP and coupl ...
Study guide test 2 Essentials of biology 2015
... • Function cellular respiration • Structures • Outer membrane • Inner membrane with cristae • Matrix with ribosomes, DNA, enzymes • 3.8 Other organelles • Lysosomes • Vacuoles • 4.1 What is energy? • Kinetic energy • Potential energy • First law of thermodynamics Conservation of energy • Entropy and ...
... • Function cellular respiration • Structures • Outer membrane • Inner membrane with cristae • Matrix with ribosomes, DNA, enzymes • 3.8 Other organelles • Lysosomes • Vacuoles • 4.1 What is energy? • Kinetic energy • Potential energy • First law of thermodynamics Conservation of energy • Entropy and ...
Chapter 2 Review Sheet Name:_______________________
... 11. Organic molecules that have the same chemical formula but different structural arrangements are called __isomers_________. 12. Carbohydrates are important because they __are the main source of energy for living things. 13. Meat, eggs, soy, and beans contain _proteins________. 14. Fruits, vegetab ...
... 11. Organic molecules that have the same chemical formula but different structural arrangements are called __isomers_________. 12. Carbohydrates are important because they __are the main source of energy for living things. 13. Meat, eggs, soy, and beans contain _proteins________. 14. Fruits, vegetab ...
chapter 2 the origin and chemistry of life
... e. The term amphiphilic describes compounds, like phospholipids, that are polar and watersoluble on one end and non-polar on the other end. 5. Steroids (Figure 2.13) a. Steroids are complex alcohols with fat-like properties. b. They are biologically important. c. Steroids include cholesterol, vitami ...
... e. The term amphiphilic describes compounds, like phospholipids, that are polar and watersoluble on one end and non-polar on the other end. 5. Steroids (Figure 2.13) a. Steroids are complex alcohols with fat-like properties. b. They are biologically important. c. Steroids include cholesterol, vitami ...
Organic Compounds Picture Vocabulary
... A compound that contains organic carbon and other atoms, usually oxygen, hydrogen, nitrogen, phosphorus, and/or sulfur. ...
... A compound that contains organic carbon and other atoms, usually oxygen, hydrogen, nitrogen, phosphorus, and/or sulfur. ...
Bio102 Problems
... 12. Phosphofructokinase (PFK) is a key enzyme in glycolysis and is heavily regulated. Which one mechanism listed below is NOT used to regulate PFK? A. PFK can be allosterically activated by AMP. B. PFK can be phosphorylated by AMPK. C. More PFK can be produced by increasing transcription of the PFK ...
... 12. Phosphofructokinase (PFK) is a key enzyme in glycolysis and is heavily regulated. Which one mechanism listed below is NOT used to regulate PFK? A. PFK can be allosterically activated by AMP. B. PFK can be phosphorylated by AMPK. C. More PFK can be produced by increasing transcription of the PFK ...
Review Problems week 11 plus any problems left over from last week
... 9) Inhibition of a key enzyme activity by the end product of a biosynthetic pathway is known as what? 10) Why is it useful to have multiple isozymes of enzymes that comprise common pathways to multiple amino acids? 11) Partial inhibition of a key enzyme activity by multiple compounds derived from an ...
... 9) Inhibition of a key enzyme activity by the end product of a biosynthetic pathway is known as what? 10) Why is it useful to have multiple isozymes of enzymes that comprise common pathways to multiple amino acids? 11) Partial inhibition of a key enzyme activity by multiple compounds derived from an ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.