Chapter 12 Section 1
... molecules were exposed to UV radiation and lightning, which provided energy for further reactions ...
... molecules were exposed to UV radiation and lightning, which provided energy for further reactions ...
Dehydration Synthesis
... Cholesterol maintains the __________________of a cell membrane We make cholesterol in our _____________and ________________ (animal fats) Steroid hormones direct our cells to do specialized tasks. o Sex hormones affect the growth and function of ______________________ o _________ is active in ...
... Cholesterol maintains the __________________of a cell membrane We make cholesterol in our _____________and ________________ (animal fats) Steroid hormones direct our cells to do specialized tasks. o Sex hormones affect the growth and function of ______________________ o _________ is active in ...
Chapter 5 Guided Notes
... Three of the four classes of macromolecules—carbohydrates, proteins, and nucleic acids—form chain-like molecules called polymers. ○ A polymer is _________________________________________________________________________ _________________________________________________________________________________ ...
... Three of the four classes of macromolecules—carbohydrates, proteins, and nucleic acids—form chain-like molecules called polymers. ○ A polymer is _________________________________________________________________________ _________________________________________________________________________________ ...
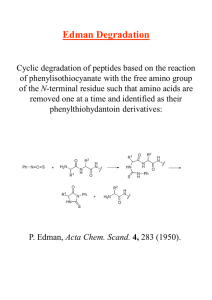
Edman Degradation
... Edman Degradation Cyclic degradation of peptides based on the reaction of phenylisothiocyanate with the free amino group of the N-terminal residue such that amino acids are removed one at a time and identified as their phenylthiohydantoin derivatives: ...
... Edman Degradation Cyclic degradation of peptides based on the reaction of phenylisothiocyanate with the free amino group of the N-terminal residue such that amino acids are removed one at a time and identified as their phenylthiohydantoin derivatives: ...
BIOENERGETICS SUMMARY File
... Products (Materials needed) (Materials produced) Photosynthesis Carbon dioxide (CO2) Glucose (C6H12O6) sugar) Water (H2O) Oxygen (O2) Cellular Respiration Sugar or other food ATP Oxygen (O2) Carbon dioxide (CO2) ...
... Products (Materials needed) (Materials produced) Photosynthesis Carbon dioxide (CO2) Glucose (C6H12O6) sugar) Water (H2O) Oxygen (O2) Cellular Respiration Sugar or other food ATP Oxygen (O2) Carbon dioxide (CO2) ...
Photosynthesis and Respiration 1. What are the three parts of an
... 10. If oxygen is not present, what process occurs in humans after glycolysis? Is it aerobic or anaerobic? Lactic acid fermentation - anaerobic 11. How are photosynthesis and cellular respiration related? Products of one produce reactants of the other ...
... 10. If oxygen is not present, what process occurs in humans after glycolysis? Is it aerobic or anaerobic? Lactic acid fermentation - anaerobic 11. How are photosynthesis and cellular respiration related? Products of one produce reactants of the other ...
Respiration Cellular respiration Redox Various Ways of Harvesting
... Energy Yield of Respiration ...
... Energy Yield of Respiration ...
Bio260 Exam1.1 MW review
... material across a cytoplasmic membrane. – Understand the different ways bacteria move material across a membrane such as facilitated diffusion and active transport mechanisms (transport systems that use proton motive force, transport systems that use ATP, and efflux pumps). ...
... material across a cytoplasmic membrane. – Understand the different ways bacteria move material across a membrane such as facilitated diffusion and active transport mechanisms (transport systems that use proton motive force, transport systems that use ATP, and efflux pumps). ...
Midterm - ltcconline.net
... A) enables the membrane to stay fluid more easily when cell temperature drops. B) enables the animal to remove hydrogen atoms from saturated phospholipids. C) enables the animal to add hydrogen atoms to unsaturated phospholipids. D) makes the membrane less flexible, allowing it to sustain greater pr ...
... A) enables the membrane to stay fluid more easily when cell temperature drops. B) enables the animal to remove hydrogen atoms from saturated phospholipids. C) enables the animal to add hydrogen atoms to unsaturated phospholipids. D) makes the membrane less flexible, allowing it to sustain greater pr ...
IIIb
... 5. (12 Pts) Unlike most organs, muscle uses three specific amino acids as energy sources. What are these amino acids (structures)? Choose one and draw its degradation pathway. ...
... 5. (12 Pts) Unlike most organs, muscle uses three specific amino acids as energy sources. What are these amino acids (structures)? Choose one and draw its degradation pathway. ...
CHAPTER 3 THE CHEMISTRY OF LIFE Section 1: Matter and
... Pure water has a pH of 7. Acidic solutions have a pH below 7, and basic solutions have a pH above 7. The pH of solutions in living things must be stable. For a stable pH to be maintained, the solutions in living things contain ...
... Pure water has a pH of 7. Acidic solutions have a pH below 7, and basic solutions have a pH above 7. The pH of solutions in living things must be stable. For a stable pH to be maintained, the solutions in living things contain ...
Sports nutrition Carbohydrates
... proteins hold together, protect, and provide structure to the body. As enzymes, hormones, antibodies, and globulins, they catalyze, regulate, and protect the body chemistry. Important biomolecules like hemoglobin, myoglobin and various lipoproteins, that carry oxygen and other substances within the ...
... proteins hold together, protect, and provide structure to the body. As enzymes, hormones, antibodies, and globulins, they catalyze, regulate, and protect the body chemistry. Important biomolecules like hemoglobin, myoglobin and various lipoproteins, that carry oxygen and other substances within the ...
Polarity
... Amino group (-NH2) Carboxyl group (-COOH) “R” group – which differs between amino acids ...
... Amino group (-NH2) Carboxyl group (-COOH) “R” group – which differs between amino acids ...
A1986A777600001
... of pyridoxal and the amino acid. The powerful electron-withdrawing ability of the N-protonated pyridine ring was also needed for catalysis. Taking some clues from the newly published Chemistry of the Metal 2Chelate Compounds, by Martell and Calvin, we quickly deduced the common mechanism for all of ...
... of pyridoxal and the amino acid. The powerful electron-withdrawing ability of the N-protonated pyridine ring was also needed for catalysis. Taking some clues from the newly published Chemistry of the Metal 2Chelate Compounds, by Martell and Calvin, we quickly deduced the common mechanism for all of ...
4. Sports nutrition, pyramid of health, healthy eating, Mediterranean
... proteins hold together, protect, and provide structure to the body. As enzymes, hormones, antibodies, and globulins, they catalyze, regulate, and protect the body chemistry. Important biomolecules like hemoglobin, myoglobin and various lipoproteins, that carry oxygen and other substances within the ...
... proteins hold together, protect, and provide structure to the body. As enzymes, hormones, antibodies, and globulins, they catalyze, regulate, and protect the body chemistry. Important biomolecules like hemoglobin, myoglobin and various lipoproteins, that carry oxygen and other substances within the ...
Chapter 5 Homework Part I. Name: Starts on page 83 What type of
... d. Which atom is a stronger “electron grabber” than almost any other type of atom? ...
... d. Which atom is a stronger “electron grabber” than almost any other type of atom? ...
Understanding Our Environment
... Chemical and Physical Bases of Life Acids and Bases • pH scale represents measurement of H+ ion ...
... Chemical and Physical Bases of Life Acids and Bases • pH scale represents measurement of H+ ion ...
Discussion Questions for Week 5: HWA Pages 167-177
... 1. Define and briefly describe the major sets of reactions in aerobic catabolism. 2. How are oxidative phrosphorylation and substrate level phosphoylation different? 3. HWA states that, in a very narrow sense, glycolysis and the Kreb’s cycle can proceed without O2. Why, then, is O2 necessary for aer ...
... 1. Define and briefly describe the major sets of reactions in aerobic catabolism. 2. How are oxidative phrosphorylation and substrate level phosphoylation different? 3. HWA states that, in a very narrow sense, glycolysis and the Kreb’s cycle can proceed without O2. Why, then, is O2 necessary for aer ...
Lecture 10 - Protein Turnover and Amino Acid
... cancers are associtated with this type of activity. ...
... cancers are associtated with this type of activity. ...
PowerPoint Presentation - Nerve activates contraction
... Storage forms of sugars (cellular fuel & some structural components) = larger size so lower solubility (doesn’t break down as easily) Starch • found in Plants • Cellulose & lignin indigestible by humans • Used for FIBER (drink lots of water!!) ...
... Storage forms of sugars (cellular fuel & some structural components) = larger size so lower solubility (doesn’t break down as easily) Starch • found in Plants • Cellulose & lignin indigestible by humans • Used for FIBER (drink lots of water!!) ...
History of Life on Earth
... • In the 1920s, Russian scientist A. I. Oparin and British scientist J.B.S. Haldane each suggested that the early Earth’s oceans once contained large amounts of organic molecules. • They both hypothesized that these molecules formed spontaneously in chemical reactions activated by energy from solar ...
... • In the 1920s, Russian scientist A. I. Oparin and British scientist J.B.S. Haldane each suggested that the early Earth’s oceans once contained large amounts of organic molecules. • They both hypothesized that these molecules formed spontaneously in chemical reactions activated by energy from solar ...
Chapter 10- Photosynthesis
... c. Each PGA then receives a phosphate from ATP plus H+ and electrons from NADPH to form PGAL. d. Two PGAL join to form a sugar phosphate, which will be modified to sucrose, starch, or cellulose. 3. Final tally= 12H2O + 6CO2 + 18ATP + 12NADPH 6O2 + C6H12O6 +18 ADP + 18 Pi + 12NADP+ +6H2O + 12H+ ...
... c. Each PGA then receives a phosphate from ATP plus H+ and electrons from NADPH to form PGAL. d. Two PGAL join to form a sugar phosphate, which will be modified to sucrose, starch, or cellulose. 3. Final tally= 12H2O + 6CO2 + 18ATP + 12NADPH 6O2 + C6H12O6 +18 ADP + 18 Pi + 12NADP+ +6H2O + 12H+ ...
Exam #2
... What are the major similarities and differences between Glycolysis and Entner-Doudoroff Pathway? Pentose Phosphate Pathway (PPP); what is its utility for a cell? How is it connected to Glycolysis, at which intermediates? Fermentation as a fate of pyruvate; endogenous organic electron acceptor; why d ...
... What are the major similarities and differences between Glycolysis and Entner-Doudoroff Pathway? Pentose Phosphate Pathway (PPP); what is its utility for a cell? How is it connected to Glycolysis, at which intermediates? Fermentation as a fate of pyruvate; endogenous organic electron acceptor; why d ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.