chapter 23
... So, when the cell needs energy, pyruvate is converted to acetyl-CoA, and the citric acid cycle proceeds. But when the cell has sufficient energy, there is not much conversion to acetyl-CoA, and the citric acid cycle slows. ...
... So, when the cell needs energy, pyruvate is converted to acetyl-CoA, and the citric acid cycle proceeds. But when the cell has sufficient energy, there is not much conversion to acetyl-CoA, and the citric acid cycle slows. ...
Organic Compounds
... Organic compounds • To build organic compounds you take hydrocarbon chains and add functional groups. • Molecules with the same functional groups will have similar physical and chemical properties. ...
... Organic compounds • To build organic compounds you take hydrocarbon chains and add functional groups. • Molecules with the same functional groups will have similar physical and chemical properties. ...
Carbohydrates What is the type of organic compound used for short
... Because they are used for insulation and protection, and they are insoluble in water. ...
... Because they are used for insulation and protection, and they are insoluble in water. ...
Metabolomic Profiling of Dynamic and Basal Measures of Glucose
... Nicholette D. Allred, PhD Associate Professor, Department of Biochemistry Dr. Allred is a molecular biologist with a research focus on understanding the genetic architecture of complex diseases and underlying risk factors in minority populations. With an emphasis on metabolic disease, her research c ...
... Nicholette D. Allred, PhD Associate Professor, Department of Biochemistry Dr. Allred is a molecular biologist with a research focus on understanding the genetic architecture of complex diseases and underlying risk factors in minority populations. With an emphasis on metabolic disease, her research c ...
Final Exam Review
... 10. Draw the reaction that produces a lipid molecule (triglyceride). What functional groups are formed as a result of the formation of a triglyceride? How many H2O are formed? 11. Identify the main components of the cell membrane on a diagram. How do the properties of each component contribute to it ...
... 10. Draw the reaction that produces a lipid molecule (triglyceride). What functional groups are formed as a result of the formation of a triglyceride? How many H2O are formed? 11. Identify the main components of the cell membrane on a diagram. How do the properties of each component contribute to it ...
Energy Unit SG Key
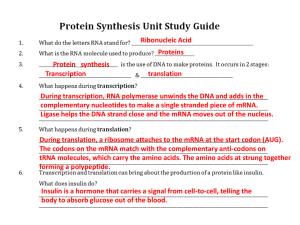
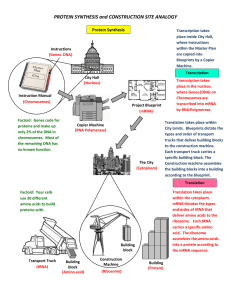
... 3 nucleotides in a row on a strand of mRNA that code for an amino acid Only mRNA The structure and function of a protein is determined by the order of the amino acids and their chemical properties. ...
... 3 nucleotides in a row on a strand of mRNA that code for an amino acid Only mRNA The structure and function of a protein is determined by the order of the amino acids and their chemical properties. ...
Natural Polymers - Wikispaces
... RNA & DNA RNA and DNA contain sugar units, making them polysaccharides, but they have well ordered groups attached to the sugars giving them unique capabilities. Messenger RNA are an example of natural polymers. These are what make possible proteins, peptides, enzymes. ...
... RNA & DNA RNA and DNA contain sugar units, making them polysaccharides, but they have well ordered groups attached to the sugars giving them unique capabilities. Messenger RNA are an example of natural polymers. These are what make possible proteins, peptides, enzymes. ...
Biomolecule Test Review 2015
... 9. What is the difference between saturated and unsaturated fatty acid? Which is better for you? Why? Saturated fatty acid- single bonds, straight and tightly packed. Solid at room temperature. (Bad for us!) Unsaturated fatty acid- double bonds bend the tails and it’s crooked (not straight). Liquid ...
... 9. What is the difference between saturated and unsaturated fatty acid? Which is better for you? Why? Saturated fatty acid- single bonds, straight and tightly packed. Solid at room temperature. (Bad for us!) Unsaturated fatty acid- double bonds bend the tails and it’s crooked (not straight). Liquid ...
outlines
... Interaction of multiple subunits (separate peptide chains) as in hemoglobin -Cooperativity Classes of Proteins 1) Globular Proteins Hydrophobic residues are sequestered in the interior of the protein while hydrophylic residues are on the outer surface. 2) Membrane Proteins Hydrophobic residues face ...
... Interaction of multiple subunits (separate peptide chains) as in hemoglobin -Cooperativity Classes of Proteins 1) Globular Proteins Hydrophobic residues are sequestered in the interior of the protein while hydrophylic residues are on the outer surface. 2) Membrane Proteins Hydrophobic residues face ...
Chapter 2 review key
... Carbohydrates—small or large carbon compounds with the empirical formula CH20. Glucose is the primary source of energy for most living things. Sugars and starches (large molecules made from simple sugars) are carbohydrates, and may be structural, such as the cellulose in plants. Proteins—are POLYMER ...
... Carbohydrates—small or large carbon compounds with the empirical formula CH20. Glucose is the primary source of energy for most living things. Sugars and starches (large molecules made from simple sugars) are carbohydrates, and may be structural, such as the cellulose in plants. Proteins—are POLYMER ...
macromolecules notes
... h. Production of water (fatty acids produce a lot of metabolic water when they are oxidized during cellular respiration). Camels’ humps are made of fat and supply water when needed. ...
... h. Production of water (fatty acids produce a lot of metabolic water when they are oxidized during cellular respiration). Camels’ humps are made of fat and supply water when needed. ...
Biomolecules Test Review -KEY
... 9. What is the difference between saturated and unsaturated fatty acid? Which is better for you? Why? Saturated fatty acid- single bonds, straight and tightly packed. Solid at room temperature. (Bad for us!) Unsaturated fatty acid- double bonds bend the tails and it’s crooked (not straight). Liquid ...
... 9. What is the difference between saturated and unsaturated fatty acid? Which is better for you? Why? Saturated fatty acid- single bonds, straight and tightly packed. Solid at room temperature. (Bad for us!) Unsaturated fatty acid- double bonds bend the tails and it’s crooked (not straight). Liquid ...
05 Cliff Note Version
... really good idea for you to watch it. Why? – The more ways you reinforce the topic, the more likely you are to transfer it to long-term memory. – You might pick up something from him that you didn’t from me. – Yeah, it looks long, but the “meat” of the video doesn’t ...
... really good idea for you to watch it. Why? – The more ways you reinforce the topic, the more likely you are to transfer it to long-term memory. – You might pick up something from him that you didn’t from me. – Yeah, it looks long, but the “meat” of the video doesn’t ...
BCHM 562, Biochemistry II
... than that of ATP. Thus, it can be used to power the ATP synthesis from ADP. This is called substrate-level phosphorylation because the phosphate donor is a Substrate with high phosphoryl-transfer potential. ...
... than that of ATP. Thus, it can be used to power the ATP synthesis from ADP. This is called substrate-level phosphorylation because the phosphate donor is a Substrate with high phosphoryl-transfer potential. ...
CELL RESPIRATION
... • Cells that contain mitochondria (Eukaryotes) normally carry out Aerobic Cell Respiration. • Pyruvate produced by Glycolysis enters the mitochondria and is catabolised by the Kreb’s Cycle and Electron Transport Chain. • The latter process requires the presence of free oxygen. ...
... • Cells that contain mitochondria (Eukaryotes) normally carry out Aerobic Cell Respiration. • Pyruvate produced by Glycolysis enters the mitochondria and is catabolised by the Kreb’s Cycle and Electron Transport Chain. • The latter process requires the presence of free oxygen. ...
Document
... •internal framework of the cell •gives the cytoplasm flexibility and strength •provides the cell with mechanical support •gives the cell its shape •can be rapidly disassembled in one area of the cell and reassembled in another •anchorage points for organelles and cytoplasmic ...
... •internal framework of the cell •gives the cytoplasm flexibility and strength •provides the cell with mechanical support •gives the cell its shape •can be rapidly disassembled in one area of the cell and reassembled in another •anchorage points for organelles and cytoplasmic ...
respiration
... If it were one simple step, all the energy would be released at once and most would be released as heat or light. This would not provide the cell with the continuous supply of energy the it needs. ...
... If it were one simple step, all the energy would be released at once and most would be released as heat or light. This would not provide the cell with the continuous supply of energy the it needs. ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.