A Voyage through Equations
... 1. Sodium combines with chlorine to produce sodium chloride. 2. When solid copper reacts with aqueous silver nitrate, the products are aqueous copper(II) nitrate and silver metal. 3. Solid iron (III) oxide and carbon monoxide react to produce iron metal and carbon dioxide gas. 4. Sulfuric acid and s ...
... 1. Sodium combines with chlorine to produce sodium chloride. 2. When solid copper reacts with aqueous silver nitrate, the products are aqueous copper(II) nitrate and silver metal. 3. Solid iron (III) oxide and carbon monoxide react to produce iron metal and carbon dioxide gas. 4. Sulfuric acid and s ...
Stoichiometry Notes
... IV. Limiting Reactants- the reactant that limits the amounts of the other reactants that can combine and the amount of the product that can form in a chemical reaction. The substance that is not used up completely in a reaction is called the excess reactant. Think about making a hamburger: let’s say ...
... IV. Limiting Reactants- the reactant that limits the amounts of the other reactants that can combine and the amount of the product that can form in a chemical reaction. The substance that is not used up completely in a reaction is called the excess reactant. Think about making a hamburger: let’s say ...
semester i - Pt. Ravishankar Shukla University
... Vector quantities and their properties Complex numbers and Coordinate transformation. Differential and Integral Calculus, Basis rules of differentiation and Integration Applications. B. The Schrodinger equation and postulates of quantum mechanics. Discussion of solutions of the Schrodinger equation ...
... Vector quantities and their properties Complex numbers and Coordinate transformation. Differential and Integral Calculus, Basis rules of differentiation and Integration Applications. B. The Schrodinger equation and postulates of quantum mechanics. Discussion of solutions of the Schrodinger equation ...
Chemistry 120
... Most reactions take place at constant pressure and therefore we define a new function, which is a state function in the same way that U is a state function ...
... Most reactions take place at constant pressure and therefore we define a new function, which is a state function in the same way that U is a state function ...
Pirogov National Medical Univercity of Vinnitsa
... places them in the workplace. 3. In the chemical laboratory, it is permitted to work only when there is a white robe and cap. Each student is given a permanent job, which he should keep clean, do not clutter up his foreign objects that are unrelated to this study. Disorder and carelessness in carryi ...
... places them in the workplace. 3. In the chemical laboratory, it is permitted to work only when there is a white robe and cap. Each student is given a permanent job, which he should keep clean, do not clutter up his foreign objects that are unrelated to this study. Disorder and carelessness in carryi ...
Ignition Processes in Hydrogen
... Ignition processes in the hydrogen-oxygen system were simulated by solving the corresponding conservation equations (i.e., conservation of mass, energy, momentum, and species mass) for one-dimensional geometries using a detailed reaction mechanism and a mUltispecies transport model. An additional so ...
... Ignition processes in the hydrogen-oxygen system were simulated by solving the corresponding conservation equations (i.e., conservation of mass, energy, momentum, and species mass) for one-dimensional geometries using a detailed reaction mechanism and a mUltispecies transport model. An additional so ...
Principles of Energy Conversion
... typically expressed in units of energy per volume (molar) or energy per mass. Conversion of chemical energy is the most important to society because this includes chemical conversion to thermal energy (combustion) and chemical conversion from electromagnetic energy (photosynthesis). If energy is rel ...
... typically expressed in units of energy per volume (molar) or energy per mass. Conversion of chemical energy is the most important to society because this includes chemical conversion to thermal energy (combustion) and chemical conversion from electromagnetic energy (photosynthesis). If energy is rel ...
Плеханов В
... atoms occurred. Thus there is a change in the effective charges on atoms or molecular fragments. In accordance with the valence bond method the bond between atoms forms using common electron pair. Covalent bond contribution is significantly great even at great difference in atoms electronegativities ...
... atoms occurred. Thus there is a change in the effective charges on atoms or molecular fragments. In accordance with the valence bond method the bond between atoms forms using common electron pair. Covalent bond contribution is significantly great even at great difference in atoms electronegativities ...
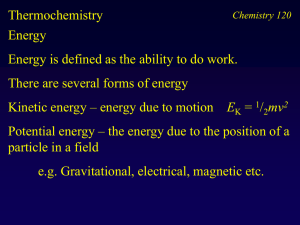
Thermochemistry Energy Energy is defined as the ability to do work
... ? UV = q V - P? V ? UV = q V + 0 = q V When the system can do PV work, i.e. a system at constant pressure, ? UP = q P - P? V where w = - P? V ...
... ? UV = q V - P? V ? UV = q V + 0 = q V When the system can do PV work, i.e. a system at constant pressure, ? UP = q P - P? V where w = - P? V ...
chemistry (9189)
... industrial and laboratory visits relevant to the content of the options chosen. In order to specify the syllabus as precisely as possible and also to emphasise the importance of skills other than recall, learning Outcomes have been used throughout. Each part of the syllabus is specified by a Content ...
... industrial and laboratory visits relevant to the content of the options chosen. In order to specify the syllabus as precisely as possible and also to emphasise the importance of skills other than recall, learning Outcomes have been used throughout. Each part of the syllabus is specified by a Content ...
2015 Dr. Jay L. Wile, All rights reserved.
... e. Several grams of magnesium, which is one of the two simplest substances produced when magnesium oxide breaks down. f. The magnesium oxide from which the magnesium discussed above was produced. g. A strawberry h. A cup of tea with no leaves in it. 3. A student does a chemical reaction with two che ...
... e. Several grams of magnesium, which is one of the two simplest substances produced when magnesium oxide breaks down. f. The magnesium oxide from which the magnesium discussed above was produced. g. A strawberry h. A cup of tea with no leaves in it. 3. A student does a chemical reaction with two che ...
1.4 Enthalpy
... 6) Methane is used in the homes as a gas supply for heating and has an enthalpy change of combustion, DcHq = -882 Kj mol-1 a) Write a balance chemical equation for the enthalpy change of combustion of methane. b) What is the enthalpy change of combustion when 160g of methane undergoes complete ...
... 6) Methane is used in the homes as a gas supply for heating and has an enthalpy change of combustion, DcHq = -882 Kj mol-1 a) Write a balance chemical equation for the enthalpy change of combustion of methane. b) What is the enthalpy change of combustion when 160g of methane undergoes complete ...
Midterm Practice Exam Key
... that corresponds to the question. DO NOT write your answers directly on the examination. Using a term from the word bank provided below, complete each of the statements that follow. Each blank is worth one mark; therefore, some questions have a total value of two marks. There are MORE terms provided ...
... that corresponds to the question. DO NOT write your answers directly on the examination. Using a term from the word bank provided below, complete each of the statements that follow. Each blank is worth one mark; therefore, some questions have a total value of two marks. There are MORE terms provided ...
Physical Science Standards
... At Level 2, the student is able to SPI interpret a distance-time graph for velocity or a velocity-time graph for acceleration, given the appropriate graph. TPI collect data and construct, analyze, and interpret graphs pertaining to distance, speed, velocity, and time. SPI solve application problems ...
... At Level 2, the student is able to SPI interpret a distance-time graph for velocity or a velocity-time graph for acceleration, given the appropriate graph. TPI collect data and construct, analyze, and interpret graphs pertaining to distance, speed, velocity, and time. SPI solve application problems ...
Elements, Compounds, and Chemical Equations
... Examine the subscripts and coefficients • Subscripts describe the number of that type of atom. They appear after the element symbol, are small, and written hanging below the ...
... Examine the subscripts and coefficients • Subscripts describe the number of that type of atom. They appear after the element symbol, are small, and written hanging below the ...
The Reaction Rates of O2 with Closed-Shell and Open
... atmosphere of about 10−8 mbar in an ion cyclotron resonance (ICR) trap do not form Al9− species even after about 600 s.18 In order to show that this hindered Al13− + O2 reaction is not just a special case but is of general interest, we measured the rate coefficients of O2 reactions with a number of Al ...
... atmosphere of about 10−8 mbar in an ion cyclotron resonance (ICR) trap do not form Al9− species even after about 600 s.18 In order to show that this hindered Al13− + O2 reaction is not just a special case but is of general interest, we measured the rate coefficients of O2 reactions with a number of Al ...
Vann - Chemistry ch. 6.1
... Suppose that a person exerts a force on the wheelbarrow that is initially at rest, causing it to move over a certain distance. Recall that the work done on the wheelbarrow by the person is equal to the product of the person's force multiplied by the distance traveled by the wheelbarrow. Notice that ...
... Suppose that a person exerts a force on the wheelbarrow that is initially at rest, causing it to move over a certain distance. Recall that the work done on the wheelbarrow by the person is equal to the product of the person's force multiplied by the distance traveled by the wheelbarrow. Notice that ...
In_Class_Practice Chapter 17 PreAP
... Calculate Keq for this equilibrium using the data [NOBr] = 0.0474 mol/L, [NO] = 0.312 mol/L, and [Br2] = 0.259 mol/L. Practice Problems 3. The following is the chemical equation for the decomposition of formamide. HCONH2(g) NH3(g) + CO(g) Calculate Keq using the equilibrium data [HCONH2] = 0.0637 ...
... Calculate Keq for this equilibrium using the data [NOBr] = 0.0474 mol/L, [NO] = 0.312 mol/L, and [Br2] = 0.259 mol/L. Practice Problems 3. The following is the chemical equation for the decomposition of formamide. HCONH2(g) NH3(g) + CO(g) Calculate Keq using the equilibrium data [HCONH2] = 0.0637 ...
2013-2014
... If the reaction was complete in 2.5 minutes, which of the following, when plotted against time, would give a graph like the one shown above? A. Total mass of the conical flask and its contents ...
... If the reaction was complete in 2.5 minutes, which of the following, when plotted against time, would give a graph like the one shown above? A. Total mass of the conical flask and its contents ...
Conductometric and Potentiometric Determination of the Solubility
... of 0.01±0.001 M distigmine bromide (DsBr2) solution to 100 ml of each of the following: 0.0033±0.001 M PMA, 0.0033±0.001 M PTA, 0.0025±0.001 M SMA, 0.0025±0.001 M STA, 0.01±0.001 M NaTPB, 0.01±0.001 M AmmRein, and 0.01±0.001 M PiH. The obtained precipitates were washed thoroughly with distilled wate ...
... of 0.01±0.001 M distigmine bromide (DsBr2) solution to 100 ml of each of the following: 0.0033±0.001 M PMA, 0.0033±0.001 M PTA, 0.0025±0.001 M SMA, 0.0025±0.001 M STA, 0.01±0.001 M NaTPB, 0.01±0.001 M AmmRein, and 0.01±0.001 M PiH. The obtained precipitates were washed thoroughly with distilled wate ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.