The First Steps of Chemical Evolution towards the
... Gcatastrophic eventsF by short circuits, population collapse, or so called Gselfish RNAF which all would limit the possibility of a higher organization proceeding through these Ghyper-cyclesF [25]. There is still another very striking argument which was proposed by Dyson [26], with his model calcula ...
... Gcatastrophic eventsF by short circuits, population collapse, or so called Gselfish RNAF which all would limit the possibility of a higher organization proceeding through these Ghyper-cyclesF [25]. There is still another very striking argument which was proposed by Dyson [26], with his model calcula ...
Chem 400 Inorganic Chemistry Laboratory
... π electrons. Two molecules of this anion will react with iron(II) to give ferrocene, the most common member of the class of organometallic compounds referred to as metallocenes. In this centrosymmetric sandwich-type π complex, all carbon atoms are equidistant from the iron atom. The extraordinary st ...
... π electrons. Two molecules of this anion will react with iron(II) to give ferrocene, the most common member of the class of organometallic compounds referred to as metallocenes. In this centrosymmetric sandwich-type π complex, all carbon atoms are equidistant from the iron atom. The extraordinary st ...
Science
... 11. Students shall relate the physical properties as they relate to different types of bonding. Stoichiometry 12. Students shall understand the relationship between balanced chemical equations and mole relationships. 13. Students shall understand the mole concept and Avogadro’s number. 14. Students ...
... 11. Students shall relate the physical properties as they relate to different types of bonding. Stoichiometry 12. Students shall understand the relationship between balanced chemical equations and mole relationships. 13. Students shall understand the mole concept and Avogadro’s number. 14. Students ...
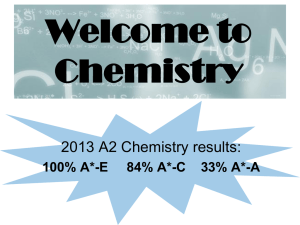
Welcome to Chemistry
... A numerate subject such as CHEMISTRY is useful for… • Accountancy/Business • Architecture • Law ...
... A numerate subject such as CHEMISTRY is useful for… • Accountancy/Business • Architecture • Law ...
Synthesis of separation processes by using case-based
... 3.5. Case-based 6s. rule-based system Rule-based systems have been the most commonly used approach to process synthesis. This kind of knowledge can also be included in a case-based reasoning system by defining ‘general cases’. For instance, if relative volatility is larger than 1.5 and the decomposi ...
... 3.5. Case-based 6s. rule-based system Rule-based systems have been the most commonly used approach to process synthesis. This kind of knowledge can also be included in a case-based reasoning system by defining ‘general cases’. For instance, if relative volatility is larger than 1.5 and the decomposi ...
NMR and Parity Violation Anomalous Temperature Dependence in
... Life is based on L-amino acids and D-sugars rather than the enantiomeric D-amino acids and L-sugars. This broken symmetry is now believed to be a feature of fundamental physics a result of symmetry-breaking induced by the weak force, which makes one enantiomer slightly more stable than the other. ...
... Life is based on L-amino acids and D-sugars rather than the enantiomeric D-amino acids and L-sugars. This broken symmetry is now believed to be a feature of fundamental physics a result of symmetry-breaking induced by the weak force, which makes one enantiomer slightly more stable than the other. ...
1 Mole
... Sometimes polyatomic ions break apart in a chemical reaction and sometimes they do not e.x. sulfate appears on both sides of the reaction so SO4 can be treated like one atom: Mg(s) + CuSO4(aq) MgSO4(aq) + Cu(s) e.x. carbonate breaks apart so atoms must ...
... Sometimes polyatomic ions break apart in a chemical reaction and sometimes they do not e.x. sulfate appears on both sides of the reaction so SO4 can be treated like one atom: Mg(s) + CuSO4(aq) MgSO4(aq) + Cu(s) e.x. carbonate breaks apart so atoms must ...
File
... catalyst while keeping other concentrations and conditions the same, and obtained the results below. Composition by volume of mixture / cm3 ...
... catalyst while keeping other concentrations and conditions the same, and obtained the results below. Composition by volume of mixture / cm3 ...
chemistry module p
... their nucleus. From the atomic mass above it could be concluded that a chlorine nucleus contains 18.45 neutrons; but there is no such thing as 0.45 of a neutron. An atomic mass, as appears on the periodic table is an average mass of all isotopes occurring in nature. This may have decimal places A ma ...
... their nucleus. From the atomic mass above it could be concluded that a chlorine nucleus contains 18.45 neutrons; but there is no such thing as 0.45 of a neutron. An atomic mass, as appears on the periodic table is an average mass of all isotopes occurring in nature. This may have decimal places A ma ...
Class XI Physical Chemistry Short note
... These neutral particles were discovered by Chadwick in 1932 when he bombarded a thin foil of Beryllium metal with fast moving alpha particles. Each particle was found to carry a mass of 1.675x10-24 g which is nearly the same as that of proton but no charge. These particles were named as neutrons. AT ...
... These neutral particles were discovered by Chadwick in 1932 when he bombarded a thin foil of Beryllium metal with fast moving alpha particles. Each particle was found to carry a mass of 1.675x10-24 g which is nearly the same as that of proton but no charge. These particles were named as neutrons. AT ...
to view
... A3. Ferromagnetic substance would make better magnets because when ferromagnetic substance is placed in magnetic field all the domains get oriented in the directionof the magnetic field and a strong magnetic effect is produced eg : Co, Ni Q4. In a compound nitrogen atoms (N) make ccp and metal atoms ...
... A3. Ferromagnetic substance would make better magnets because when ferromagnetic substance is placed in magnetic field all the domains get oriented in the directionof the magnetic field and a strong magnetic effect is produced eg : Co, Ni Q4. In a compound nitrogen atoms (N) make ccp and metal atoms ...
Abstraction and its Limits: Finding Space For Novel Explanation
... that the explanations given by one theory may be deemed novel with respect to another theory. When we properly understand the role of abstraction, we appreciate that explanatory value may be irreducible, even where theoretical reduction is possible. Particular kinds of complex relationships between ...
... that the explanations given by one theory may be deemed novel with respect to another theory. When we properly understand the role of abstraction, we appreciate that explanatory value may be irreducible, even where theoretical reduction is possible. Particular kinds of complex relationships between ...
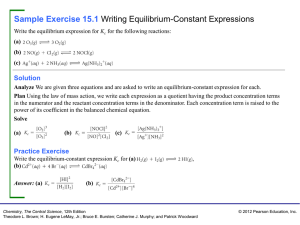
Sample Exercise 15.1 Writing Equilibrium
... Plan For equilibrium to be achieved, it must be possible for both the forward process and the reverse process to occur. For the forward process to occur, there must be some calcium carbonate present. For the reverse process to occur, there must be both calcium oxide and carbon dioxide. In both cases ...
... Plan For equilibrium to be achieved, it must be possible for both the forward process and the reverse process to occur. For the forward process to occur, there must be some calcium carbonate present. For the reverse process to occur, there must be both calcium oxide and carbon dioxide. In both cases ...
Chapter 1 Introduction: Matter and Measurement
... For a Fe metal object whose density is 7.86 g/mL. (a) What is the mass (g) of a piece of this metal if it displaces 12. mL of water in a graduated cylinder? ...
... For a Fe metal object whose density is 7.86 g/mL. (a) What is the mass (g) of a piece of this metal if it displaces 12. mL of water in a graduated cylinder? ...
mass mass calc
... C(s) + O2(g) CO2(g) In a particular lab set up, 50.0 g of oxygen gas are available for the combustion of 25.0 g of carbon.. a) Calculate the number of moles of oxygen gas and carbon solid that are each available to react. b) Calculate the number of moles of oxygen gas that will actually be needed ...
... C(s) + O2(g) CO2(g) In a particular lab set up, 50.0 g of oxygen gas are available for the combustion of 25.0 g of carbon.. a) Calculate the number of moles of oxygen gas and carbon solid that are each available to react. b) Calculate the number of moles of oxygen gas that will actually be needed ...
The Equilibrium Constant K
... that for the reaction written in reverse. When the balanced equation for a reaction is multiplied by a factor of n, the equilibrium expression for the new reaction is the original expression raised to the nth power; thus Knew = (Koriginal)n. K values are usually written without units. ...
... that for the reaction written in reverse. When the balanced equation for a reaction is multiplied by a factor of n, the equilibrium expression for the new reaction is the original expression raised to the nth power; thus Knew = (Koriginal)n. K values are usually written without units. ...
Study on Halide Ions Selectivity of Industrial Grade Anion
... anion exchange resin Auchlite A-378 in chloride form towards iodide and bromide ions in the solution. The study was conducted by performing the Clˉ/Iˉ and Clˉ/Brˉ uni-univalent ion exchange reactions under gradually increasing temperature conditions. The thermodynamic equilibrium constants K values ...
... anion exchange resin Auchlite A-378 in chloride form towards iodide and bromide ions in the solution. The study was conducted by performing the Clˉ/Iˉ and Clˉ/Brˉ uni-univalent ion exchange reactions under gradually increasing temperature conditions. The thermodynamic equilibrium constants K values ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.