* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 14.1 Dynamic Equilibrium, Keq , and the Mass Action Expression
Electrolysis of water wikipedia , lookup
Chemical potential wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Electrochemistry wikipedia , lookup
Asymmetric induction wikipedia , lookup
Marcus theory wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Thermomechanical analysis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Thermodynamics wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Process chemistry wikipedia , lookup
Thermodynamic equilibrium wikipedia , lookup
Hydroformylation wikipedia , lookup
George S. Hammond wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Chemical reaction wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Rate equation wikipedia , lookup
Crystallization wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Click chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
Transition state theory wikipedia , lookup
14.1 Dynamic Equilibrium, Keq ,
and the Mass Action Expression
The Equilibrium Process
Dr. Fred Omega Garces
Chemistry 201
Miramar College
1
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
Extent of a Reaction
Chemical Reaction
Most reactions do not occur with 100% conversion to products. At the
molecular, when a reaction occurs to form products, some products
will back react to form reactants.
The extent of the reaction i.e., 20%
or 80% can be determine by
measuring concentration of each
component in solution
In general the extent of the reaction
is a function of the following:
Temperature, concentration and degree of organization, all of
which are monitored by some constant value called the –
The equilibrium constant, Keq
2
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
How Equilibrium is achieve
Consider the reaction A D B
Forward
A g B
ratef = kf[A]
Reverse
B f A
rateb = kb[B]
Overall
A D B
rate forward kf[A] = rate reverse kb[B]
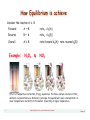
Example:
N2O4 D
NO2
Effect of temperature on the NO2 D N2O4 equilibrium. The tubes contain a mixture of NO2
and N2O4. As predicted by Le Chatelier’s principle, the equilibrium favors colorless N2O4 at
lower temperatures. but shifts to the darker brown NO2 at higher temperature.
3
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
Equilibrium: Mass Action Expression
When equilibrium is establish, A D B
“D” illustrates that rate forward = rate reverse
or Kf[A] = Kb[B]
– Rearranging this equation yields Kf/Kb = [B] / [A] which yields
– The mass action expression : Keq = [B] / [A]
For any generic chemical process at equilibrium
aA + bB D pP + qQ
A mass action expression can be written:
p
q
P ] ∗ [Q ]
[
K eq =
a
b
[A] ∗ [B]
This is also referred to as the Law of Mass Action
4
€
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
Haber Process
Consider the Haber Process:
N2 (g) + 3H2
(g)
D 2NH3 (g)
(not 100 % process)
As soon as NH3 is form, it back reacts and forms N2 and H2.
Law of Mass Action
Or
Mass Action Expression
2
K eq =
[NH3 ]
3
[N2 ] ∗ [H2 ]
= Kc
€
5
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
Meaning of Keq : Sidebar
Review of Fractions
x
y
< 1
: If x < y
x
y
> 1
: If x > y
x
y
= 1
: If x = y
the denominator dominates.
€
B) For x > y,€ the numerator dominates.
C) For x = y, numerator and denominator are equal.
A) For x < y,
€
6
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
Meaning of Keq
Which is favored, Reactant or Product?
p
k eq
€
q
x
P ] • [Q ]
Product]
[
[
=
=
[A] • [B] [Reactant]
A
b
y
A) For Keq < 1, the ratio [Product]x < [Reactant]y
B) For Keq > 1, the ratio [Product]x > [Reactant]y
C) For Keq = 1, the ratio [Product]x = [Reactant]y
A) For Keq < 1, at equilibrium Reactant is favored.
B) For Keq > 1, at equilibrium Product is favored.
C) For Keq = 1, at equilibrium Product and Reactant are equal.
7
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
Understanding the Concept
1. What is the distinction between rates and the extent
of the reaction?
2. What does the term dynamic equilibrium mean
physically and at the atomic level?
3. What does the magnitude of Keq mean in terms of the
chemical reaction?
4. If there is no change in concentration of reactants
and product at equilibrium, why is it considered
dynamic?
8
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
For Reactions Not at Equilibrium
For reactions not yet at equilibrium, the Law of Mass action yield
information in terms of the Reaction Quotient.
Consider the following chemical process not at equilibrium.
aA + bB D
rR + pP
A reaction quotient expression can be written:
[R]r [P]p
Q=
[A]a [B]b
Where the numerical value of Q will determine the direction the
reaction will proceed.
Q < Keq
€Q > K
eq
9
Reaction shifts to g Right
Reaction shifts to f Left
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
LeChatelier’s Principle (Preview)
Concentration Effect
If a chemical system is at equilibrium and then a substance is added (either a
reactant or product), the reaction will shift so as to re-establish equilibrium by
subtracting part of the added substance. Conversely, removal of a substance
will result in the reaction moving in the direction that forms more of the
substance.
Consider the Haber reaction that
was discussed earlier.
N2 + 3H2 D 2NH3 + E
If some H2 is added to the reaction
which was at equilibrium, the system
self-adjust to remove the excess H2
by converting it to NH3 until
equilibrium is re-establish; in the
process some N2 is also consumed.
10
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
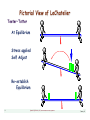
Pictorial View of LeChatelier
Teeter•Totter
At Equilibrium
Stress applied
Self Adjust
Re-establish
Equilibrium
11
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
Keq and the Q
The change in Q during a reaction and its relation to Keq:
Consider the reaction:
N2O4 D 2 NO2
The reaction coordinate diagram shows how the
concentration of N2O4 and NO2 changes as the reaction
approaches equilibrium. This is also reflected in Q. As
the reaction proceeds to the right, N2O4 to NO2, the Q
value increases, N2O4 becomes smaller and NO2
becomes larger. The reaction finally reaches
equilibrium at teq, at which time the concentration of
N2O4 and NO2 remains constant. Equilibrium is reached
and the reaction quotient becomes equal to the
equilibrium constant. Q = Keq.
12
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
Heterogeneous Systems
The equilibrium of substances with different phases.
i.e., solid and aqueous coexisting Which solid is more concentrated?
Solid and gas coexisting Which solid is more concentrated?
Concentration of a solid is always a Constant;
Even though the two containers contain different amounts of solid
solute, as long as both solids are present at a given temperature,
A) the concentration of the solution is the same, B) the partial
pressure of the gas is the same.
Concentration of solid remains the same (constant) !
The solid component is not taken into account in the
Mass Action expression or the Reaction Quotient.
13
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
Heterogeneous Systems CaCO3
Consider the following reaction:
CaCO3(s) D CaO (s) + CO2 (g)
k eq =
but,
[CaO] ∗ [CO2 ]
[CaCO3 ]
[CaO] and [CaCO3 ]
Constant1 ] ∗ [CO2 ]
[
k eq =
[Constant2 ]
k eq = [CO2 ]
€
14
= Constant
The Mass Action Expression for a heterogenerous system need not take into account
the concentration of the solid. Even though
the two containers have different amount of
solids (CaCO3 and CaO), as long as both solids
are present at the same temperature, the
partial pressure of CO2 is the same at
equilibrium.
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
In Class exercise
The reaction is in a 10.0 L vessel. The initial concentration of [H2] is 12.0 molar and that of [N2] is 4.0 molar.
i) Sketch the change of the concentration of N2 from 0 to 3.5 min. T = 200 K
ii) At 2 minutes, Q is:
a) increasing
b) decreasing
c) not changing
d ) cannot be determine
iii) At 5 minutes, Q is: a) increasing
b) decreasing
c) not changing
d ) cannot be determine
iv) Calculate the equilibrium constant at the 4.0-minute mark.
2.1•10-2 M-2
v) Calculate the equilibrium constant at the 8.0-minute mark.
2.1•10-2 M-2
15
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
Keq as a Function of the Written Reaction
What is the equilibrium constant if a reaction is written in the reverse direction?
Consider,
COCl2
and the reverse reaction
CO (g) + Cl2 (g)
(g)
D CO (g) + Cl2 (g)
D
COCl2
(i)
(ii)
(g)
What is the relationship of Keq between both reaction (i) & (ii)?
K eq (i) =
[CO] [Cl2 ]
[COCl2 ]
Inverse relationship -
K eq (i) = K eq (ii)
[COCl2 ]
K eq (ii) =
[CO] [Cl2 ]
hence
[CO] [Cl2 ] " [COCl2 ] %
=$
[COCl2 ] # [CO] [Cl2 ] '&
€
16
€
K forward =
−1
Dynamic Equilibrium , Keq and the Mass Action Expression
-1
1
K reverse
January 13
Effects of Keq and Variation of a Chem Equation
Consider the variation of a chemical reaction:
A D B
Keq = [B] / [A]
B D A
Krev = [A] / [B]
Krev = 1 / Keq
K’
= [B]1/2 / [A]1/2
K’ = (Keq)1/2
K”
= [A]2 / [B]2
K ” = (Keq)-2
1/2
A D
1/2
2B D 2A
n
A D
Note:
17
n
B
B
K’ = [B] n / [A] n K’ = (Keq)
n
Krev = 1 / Keq
or
Keq = 1 / Krev
K’ = (Keq)1/2
or
Keq = K’2
K ” = (Keq)-2
or
Keq = (K ” )-1/2
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
Expressing Q and Calculating K
The relationship of the Mass Expression, Keq,
can be extended to the Reaction Quotient, Q:
18
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
Example 2:
Keq relationship
Consider the following equilibrium, at 480 °C:
2Cl2(g) + 2H2O (g) D 4 HCl(g) + O2 (g)
Kp = 0.0752
a) What is the value of Kp for: 4 HCl(g) + O2 (g) D 2Cl2(g) + 2H2O (g)
b) What is the value of Kp: Cl2(g) + H2O (g) D 2HCl(g) + 1/2 O2 (g)
c) What is the value of Kc: 2 Cl2(g) + 2H2O (g) D 4HCl(g) + O2 (g)
(To be discussed later)
a) This equation is the reverse of the original
b) This equation is half the original
c) This equation will be solve in the next series of slides.
19
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
Relationship Between Kc and Kp
Consider the Reaction:
COCl2 (g) D CO (g) + Cl2 (g)
(
p CO) * ( p Cl2 )
KP =
( p COCl2 )
or
[CO] [Cl2 ]
Kc =
[COCl2 ]
It can be shown that:
€
K c = K p (RT)
−Δn
€
K p = K c (RT)
+ Δn
[RT is positive exponent (+Δn)]
where Δn = ∑ (gas moles)
− ∑ (gas moles)
product
reactant
€
20
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
Derivation Kc and Kp relationship
[
P CO] ∗ [ P Cl2 ]
kp =
[ P COCl2 ]
and
kc
CO] ∗ [Cl2 ]
[
=
[COCl2 ]
but PV = nRT
therefore
€
kp
€
€
21
n
nRT
= RT = [conc] ∗ RT
V
V
CO] ∗ [ Cl ] {[CO] ⋅ RT} ∗ {[Cl ] ∗ RT}
[
=
=
{[COCl ] ∗ RT}
[ COCl ]
p
p
p
kp
P=
2
2
2
2
CO] ∗ [Cl2 ] RT ∗ RT [CO] ∗ [Cl2 ] RT
[
=
∗
=
∗
RT
[COCl2 ]
[COCl2 ]
[CO] ∗ [Cl2 ] ∗ RT +1 = k ∗ RT +Δn
kp =
c
[COCl2 ]
1
∴ k p = k c ∗ RT +Δn and k c = k p ∗ RT −Δn
where Δn = ∑ (gas moles)
− ∑ (gas moles)
product
reactant
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
Summary: Equilibrium constant
• The mass action expression is equal to the equilibrium constant.
• The equilibrium constant value is generally written without units.
• The equilibrium expression for a reaction is the reciprocal of that
reaction when it is written in the reverse direction.
• When the balanced equation for a reaction is multiplied by a factor
n, the equilibrium expression for the factored reaction is the
original expression raised to the nth power.
Equation 1ref: R D P,
Equation 2: nR D nP,
Thus K2 = (k1ref)n
The equilibrium constant express in terms of concentration, Kc, is
related to the equilibrium constant expressed in terms of partial
pressure, Kp, by the amount of gas moles changes during the course
of the reaction.
22
Dynamic Equilibrium , Keq and the Mass Action Expression
January 13
14.2 Solving Equilibrium Problems
The iΔe Process
Dr. Fred Omega Garces
Chemistry 201
Miramar College
1
Solving Equilibrium Problems
January 13
Solving Equilibrium Problems
There are many type of equilibrium problems which arise
in the real world and in chemistry exams. These problems
can be broken down to two basic types.
1. The first type is one in which the equilibrium concentrations are
given and the equilibrium constant must be solved.
Given: [Conc] at equilb Solve: Keq
2. The second type is one in which both the equilibrium constant and
the initial concentration are given and the concentrations at
equilibrium are solved for.
Given: [Conc]o and Keq Solve: [Conc] at equilb
2
Solving Equilibrium Problems
January 13
Type 1: Equilibrium
The first type of problem is the type in which the equilibrium
concentrations are given and the equilibrium constant, Keq, must
be solved.
Given: [Conc] at equilb
Solve: Keq
Steps involved in the equilibrium calculations.
1. Write the balanced equation for the reaction.
2. Write the equilibrium expression using the Mass Action
Expression.
3. Plug the equilibrium concentration into the Mass Action and
solve for the equilibrium constant, Keq .
3
Solving Equilibrium Problems
January 13
1.
Calculating Keq:
Kc - Kp relationship
(Type1)
Carbon dioxide is placed in a 5.00-L high-pressure reaction vessel at 1400.°C. Given the following
reaction, CO (g) + 1/2 O2 (g) D CO2 (g) , calculate the equilibrium constant Kc and Kp after equilibrium
is achieved with 4.95 mol of CO2 , 0.0500 mol of CO, and 0.0250 mol of O2 found in the container.
T = 1400°C
V = 5.0 L
CO(g)
+
i
Δ
mol e
1
2
O2 (g)
!
CO2 (g)
0.050 mol
0.025mol
4.95mol
1.00 ⋅10 -2 M
5.00 ⋅10 -3 M
9.90 ⋅10 -1 M
Vol = 5.0 L
[e]
€
Supplemental: Calculate the partial pressure of each gas at equilibrium
4
Solving Equilibrium Problems
January 13
2.
Calculating Keq iCe - method
(Type1 & 2)
A mixture of 0.1000 mol of CO2 , 0.05000 mol of H2, and 0.1000 mol of H2O is placed in a
1.000 L vessel. The following equilibrium reaction is established:
CO2 (g) + H2 (g) D CO(g)+ H2O (g)
At equilibrium [CO2] = 0.0954 M. (a) Calculate the equilibrium concentrations of H2, CO,
and H2O. (b) Calculate the Kc for the reaction (c) Calculate Kp for the reaction?
CO2(g)
V = 5.0 L
i 0.1000M
Δ
−X
[e]
0.0954
+
H2 (g)
0.0500
−X
?
!
CO
(g)
+
+X
?
H2O (g)
0.1000
+X
?
€
Supplemental: Calculate the partial pressure of each gas at equilibrium.
6
Solving Equilibrium Problems
January 13
Type 2: Equilibrium
The second type of problems provides information on both the
equilibrium constant and the initial concentration. The
concentrations at equilibrium must be solved for.
Given: [Conc]o and Keq
Solve: [Conc] at equilb
Steps involve in the equilibrium calculations.
1. Write the balanced equation for the reaction.
2. Write the equilibrium expression using the Mass Action Expression.
3. List the initial conditions.
4. Set up the iΔe table and solve for the equilibrium concentration in
terms of a variable (x).
5. Plug the equations expressing the equilibrium concentration into the
mass action expression and solve for x.
6. Assign the value of x to the equilibrium concentration equation and
determine the numerical value for the equilibrium concentration for
each specie in the reaction.
8
Solving Equilibrium Problems
January 13
3a.
Calculation: Product formed @ Equilibrium (Type2)
How much SO3 is formed when 0.50 mol of SO2 and 0.50 mol NO2 are in 1.00
L at 821 °C. Kc = 6.85 at this temperature.
SO2(g)
+ NO2(g)
i
Δ
0.50 M
−X
0.50M
−X
0.00
+X
0.00
+X
[e]
0.50 - X
0.50 - X
+X
+X
!
SO3
(g)
+ NO(g)
Supplemental: If this was a stoichiometry problem, what is the mole of SO3 when reaction is complete?
9
Solving Equilibrium Problems
January 13
3a.
Calculation: Product formed @ Equilibrium (Type2)
How much SO3 is formed when 0.50 mol of SO2 and 0.50 mol NO2 are in 1.00
L at 821 °C. Kc = 6.85 at this temperature.
10
SO2(g)
+ NO2(g)
i
Δ
0.50 M
−X
0.50M
−X
0.00
+X
0.00
+X
[e]
0.50 - X
0.50 - X
+X
+X
!
SO3
Solving Equilibrium Problems
(g)
+ NO(g)
January 13
3b.
Calculation: Product formed @ Equilibrium (Type2)
How much SO3 is formed when 0.50 mol of SO2 and 1.00 mol NO2 are in 1.00 L at 821
°C. Kc = 6.85 at this temperature. (Non-perfect square)
SO2(g)
i
Δ
[e]
12
+
NO2(g)
!
SO3
(g)
+ NO(g)
0.50 M
−X
1.00M
−X
0.00
+X
0.00
+X
0.50 - X
1.00 - X
+X
+X
Solving Equilibrium Problems
January 13
4. Calculating Keq: Method of Approximation (Type2)
NOCl decomposes to form the gases NO and Cl2. At 35°C the equilibrium constant is
Kc = 1.6•10-5. If 1.0 mol of NOCl is placed in a 2.0 L flask what are the concentration
of all specie at equilibrium?
2NOCl(g)
V = 2.0 L
14
!
2NO
+ Cl2
(g)
i
Δ
0.50 M
− 2X
0
+ 2X
0
+X
[e]
0.5 - 2X
+ 2X
+X
Solving Equilibrium Problems
(g)
K c = 1.6⋅ 10 -5
January 13
Assumption Check
When making assumptions, if a reaction has a relatively small keq and a relatively
large initial reactant concentration, then the concentration change (x) can often
be neglected without introducing significant error. This does not mean x = 0,
because then this would mean there is no reaction. It means that if a reaction
proceeds very little (small k) and if you start with a high reactant concentration,
very little will be used up, so the following holds.
[react]o -x ≈ [react]eq ≈ [react]o
When making the assumption that x is negligible, you must check that the error
introduced is not significant. If the assumption results in a change (error) in
concentration of less than 5%, the error is not significant and the assumption is
justified.
To test the assumption, use the following formula:
(Δ conc / initial concentration) • 100 < 5%
In the previous problem, the assumption is check by the following calculations:
[2x / (0.5) ] • 100 = [ 2(1•10-2) / 0.5 ]•100 = 4% < 5%
The assumption is valid in
this example.
16
Solving Equilibrium Problems
January 13
Assumption Check [React]initial / keq
In general, simplifying assumptions works when the initial
concentration of the reactant is high but not when it is low. To
summarize, we assume that x (Δ [React]) can be neglected if Keq is
small relative to [React]initial. The benchmark in justifying the
assumption isYour job is to determine what is the threshold for
[React]initial / keq so that the assumption is justified.
If [React]initial / ka > 400, the assumption is justified; neglecting x
introduces an error of < 5%
If [React]initial / ka < 400, the assumption is not justified; neglecting x
introduces an error of > 5%
17
Solving Equilibrium Problems
January 13
5. Calculating Keq:
Quadratic or iteration?
At 250 ° C, the reaction PCl5 (g) D PCl3 (g) + Cl2 (g) has an equilibrium constant
Kc = 1.80. If 0.100 mol PCl5 is added to a 5.00-L vessel, what are the
concentrations of all specie at equilibrium ? What % of the reactant remains?
PCl5
(g)
!
PCl3
(g)
+ Cl2(g)
i
Δ
0.02 M
−X
0
+X
0
+X
[e]
0.02 - X
+X
+X
18
Solving Equilibrium Problems
January 13
6. Calculation: Product formed @ Equilibrium (Type2)
Exactly 4 mol of SO3 is sealed in a 5.0 L container @1500K. Kc is
9.34•103 for the rxn, what are the conc. of all specie at equilb?
20
Solving Equilibrium Problems
January 13
7. ... Another equilibrium problem (difficult)
An engineer is studying the oxidation of SO2 to produce the precursor, SO3, in
the manufacture of sulfuric acid. The Kp = 1.7 • 108 at 600. K for the reaction.
2SO2 (g) + O2(g) D 2SO3 (g)
i) At equilibrium pSO3 = 300. atm and pO2 = 100. atm. Calculate pSO2
ii) The engineer places a mixture of 0.0040 mol of SO2(g) and 0.0028 mol of
O2(g) in a 1.0-L container and raises the temperature to 1000K. The reaction
reaches equilibrium and 0.0020 mol of SO3 (g) is present. What would the
engineer calculate the Kc and PSO2 for the reaction to be 1000K. Does the
engineer expects the reaction to be exothermic or endothermic?
22
Solving Equilibrium Problems
January 13
Procedure for Solving Equilibrium Problems
1.
2.
3.
4.
Write the balanced equation for the reaction.
Write the equilibrium expression using the Mass Action Expression.
List the initial conditions.
Determine the type of equilibrium problem you have.
Type 1 - Given the equilibrium concentration, solve for Keq .
Type 2 - Given Keq and initial concentration, solve for conc. @ equilb.
Type1
Plug the equilibrium concentration into the Mass Action and solve for the equilibrium
constant, Keq .
Type2
a) Set up the iΔe table and solve for the equilibrium concentration in terms of a
variable (x).
b) Plug the equations expressing the equilibrium concentration into the mass action
expression and solve for x
c) Assign the value of x to the equilibrium concentration equation and determine the
numerical value for the equilibrium concentration for each specie in the reaction.
23
Solving Equilibrium Problems
January 13
14.3 LeChatelier
Predicting Direction of a
Chemical Reaction
Dr. Fred Omega Garces
Chemistry 201
Two equal-weight boys at equilibrium on a
teeter totter. The boy at the right is given
a five-pound weight thereby disturbing the
equilibrium. The boy on the left scoots
farther back on the teeter totter to
restore the equilibrium.
1
LeChatlier and Chemical Direction
Miramar College
January 13
Quotient: Review
For reactions not yet at equilibrium, the Law of Mass action yield
information in terms of the Reaction Quotient.
Consider the following chemical process not at equilibrium.
aA + bB D
rR + pP
A reaction quotient expression can be written:
[R]r [P]p
Q=
a
b
[A] [B]
Where the numerical value of Q will determine the direction the
reaction will proceed.
€
2
Q < Keq
Reaction shifts to g Right
Q > Keq
Reaction shifts to f Left
LeChatlier and Chemical Direction
January 13
Reaction Quotient: Direction of Reaction
Consider, T =532 °C, Kc = 0.19 what direction will the reaction
proceed if the initial concentration is change to-
N2 (g)
i) 0.30 M
+
3H2
D
(g)
0.20 M
2NH3 (g)
0.10 M
2
0.10M]
[
Q=
[0.30M] ∗ [0.20M]
3
=
€
3
LeChatlier and Chemical Direction
January 13
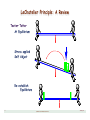
LeChatelier Principle: A Review
Teeter•Totter
At Equilibrium
Stress applied
Self Adjust
Re-establish
Equilibrium
5
LeChatlier and Chemical Direction
January 13
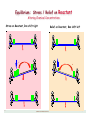
Equilibrium: Stress / Relief on Reactant
Altering Chemical Concentrations
Stress on Reactant, Rxn shift right
6
LeChatlier and Chemical Direction
Relief on Reactant, Rxn shift left
January 13
Equilibrium: Stress / Relief on Product
Altering Chemical Concentrations
Stress on Product, Rxn shift left
7
LeChatlier and Chemical Direction
Relief on Product, Rxn shift right
January 13
Exothermic Heats of Solution
Exothermic Process.
Energy is a product
Heating a solution in
which the ΔHsoln is
exothermic Energy is a
product, ΔHsoln ( - ),
results in a shift of the
reaction to the left, or
more solute
precipitating out of
solution.
8
LeChatlier and Chemical Direction
January 13
Endothermic Heats of Solution
Endothermic Process.
Energy is a reactant
Heating a solution in
which the ΔHsoln is
endothermic, Energy is
a reactant, ΔHsoln ( + ),
results in a Shift of the
reaction to the right, or
more solute dissolving
in solution.
9
LeChatlier and Chemical Direction
January 13
Solubilities of Solids Vs. Temperature
Solubility of several ionic solid as a
function of temperature.
Most salts have positive heats of
solution. When the salt solution is
heated, more solute dissolves.
Some salts have negative enthalpy
of solution, (exothermic process)
i.e., Ce2(SO4)3. When these salt
solutions are heated, the solute
becomes less soluble.
Other example:
Mg(OH)2 and Starch
10
LeChatlier and Chemical Direction
January 13
2ii) Temperature & Solubility: Gases
Temperature - (Gas)
Consider the extent in which O2 or CO2 dissolves in
water. What are the conditions which will increase the
solubility of gas in water.
As the temperature
increase, both solute and
solvent will be moving
faster, the gas solute
however will now have
enough energy to leave the
liquid interface
[Solute]
D
gas
[Solute]
Solution
Is this an exothermic or
endothermic process?
11
LeChatlier and Chemical Direction
January 13
Gas solute; Exothermic ΔHsoln
As the temperature increase,
both solute and solvent will
be move faster. The gas
solute however will now have
enough energy to leave the
liquid interface because IMF
can be overcome
12
LeChatlier and Chemical Direction
January 13
Disaster: (1700 dead)
from Gas Solubility
Lake Nyos in Cameroon, the site of a
natural disaster. In 1986 a huge bubble
of CO2 escaped from the lake and
asphyxiated more than 1700 people.
http://hubpages.com/hub/The-Exodus-Decoded
13
LeChatlier and Chemical Direction
In the African nation of Cameroon in
1986 a huge bubble of CO2 gas
escaped from Lake Nyos and moved
down a river valley at 20 m/s (about
45 mph). Because CO2 is denser than
air, it hugged the ground and
displaced the air in its path. More
than 1700 people suffocated. The
CO2 came from springs of
carbonated groundwater at the
bottom of the lake. Because the lake
is so deep, the CO2 mixed little with
the upper layers of water, and the
bottom layer became
supersaturated with CO2. When
this delicate situation was changed,
perhaps because of an earth-quake
or landslide, the CO2 came out of
the lake water just like it does when
a can of soda is opened.
January 13
The effect of a Change in Temperature
in terms of Reaction Quotient and LeChatelier
In an endothermic process, energy is a reactant and an increase in temperature results
in a shift of the reaction to the right. When the temperature is decreased the reaction
shifts to the left.
In an exothermic process, energy is a product and an increase in temperature results in
a shift of the reaction to the left. When the temperature is decreased the reaction
shifts to the right.
The question is raised, why does the reaction adjust itself, when according to the Mass
Action Expression, the concentrations of chemicals are not altered with temperature
changes? What causes the Mass Action Expression not to equal Keq(new) (≠ keq(old)).
Consider an Endothermic reaction: E + R D P: Keq = [Prod] / [React] . If
the temperature is raised, the reaction shifts to the right, (Q < Keq )
A shift to the right means that [Prod] = h (will raised) and [React] = i (will lowers).
This will only occur if the [Prod] / [React] (or Q) is now less than Keq @ new the
temperature. In other words when the temperature is change (increase T), the
equilibrium constant changes, the current [P] / [R] ratio is still equal to the old Keq which
is now Q (the reaction quotient) and the reaction shifts to re-establish equilibrium that
is to attain the new Keq.
14
LeChatlier and Chemical Direction
January 13
Temperature Effect (increase) & Reaction Quotient
Exothermic Rxn:
Increase in Temperature
Endothermic Rxn:
Increase in Temperature
K
eq(old Temp)
= Q
Direction
Reaction
Keq
Direction
Reaction
K
eq(old Temp)
= Q
@
When the temperature is
When the temperature is
New
raised for an exothermic
raised for an endothermic Temperature
reaction, the Keq constant
reaction, the Keq constant
changes. Because the
changes. Because the
current concentrations yields
current concentrations yields
a reaction quotient greater
a reaction quotient less than
than Keq (new) the reaction
Keq (new) the reaction must
must shift to the left.)
shift to the right.
15
LeChatlier and Chemical Direction
January 13
Temperature Effect (decrease) & Reaction Quotient
Exothermic Rxn:
Decrease in Temperature
K
eq(old Temp)
= Q
Direction
Reaction
Endothermic Rxn:
Decrease in Temperature
Keq
@
When the temperature is
New
lowered for an exothermic Temperature
reaction, the Keq constant
changes. Because the
current concentrations yields
a reaction quotient lower
than Keq (new) the reaction
must shift to the right.
16
LeChatlier and Chemical Direction
Direction
Reaction
K
eq(old Temp)
= Q
When the temperature is
lowered for an endothermic
reaction, the Keq constant
changes. Because the
current concentrations yields
a reaction quotient greater
than Keq (new) the reaction
must shift to the left.
January 13
Temperature Effect on
[CoCl4]2- D [Co(H2O)]
Consider the reaction:
[CoCl4]2- + 6H2O D
blue
ΔH < 0
[Co(H2O)] + 4Clpink
In this experiment when the solution was placed in cold water, the
solution turned vivid pink. The pink color indicates a shift to the right for
the reaction shown above. This will only occur if the new Keq at the instant
the temperature is altered, is now higher than the old Keq, (which is now
called Q). Therefore the reactant concentration decreases, the product
concentration increases as the reaction adjust itself so that the Mass
action equals Keq: Q g Keq
K
17
eq(old Temp)
= Q
Direction
Reaction
LeChatlier and Chemical Direction
K
eq (new Temp)
January 13
3i) Pressure on Solubility:
Solids / Liquid
Pressure - (Solid and Liquid)
The solubility of solids and liquids are hardly
affected by pressure.
Solids and liquids are
already very close to
each other. An
increase in pressure will
not affect solubility.
18
LeChatlier and Chemical Direction
January 13
3 ii) Pressure on Solubility: Gas
Pressure - (Gas)
Solubility of gas is greatly affected by pressure
Henry’s Law: PA = KHXA
Gas solutes are very sensitive to
pressure. Because gas particles are
separated by void space, an increase
in pressure will increase the
solubility of the gas.
Divers must be careful when diving
to great depths because the
potential of dissolved N2 gas in the
blood will lead to the bends.
19
LeChatlier and Chemical Direction
January 13
Pressure Affect:
Teeter Totter Analogy
[Solute]
Pressure
Sensitive
D
gas
[Solute]
Solution
In utilizing LeChatelier
Principle to determine the
direction of solubility for a
gaseous solute with variation in
pressure, the first thing that
must be establish is which side
is more sensitive to pressure.
In our case the gas is more
sensitive than the solution.
20
LeChatlier and Chemical Direction
January 13
The effect of a Change Volume of reaction vessel
in terms of Reaction Quotient and LeChatelier
For a chemical reaction, LeChatelier Principle can be verified in terms of the Reaction
Quotient. Consider the reaction: N2 + 3H2 D 2NH3
Let [H2] = 0.1207 M, [N2] = 0.0402 M and [NH3] = 0.00272 M for a 1-L vessel at equilibrium.
In which direction will the reaction shift if the reaction vessel is decreased by half such that
V = Vo/2. The concentrations will now double for all specie at the instant the volume is
decreased. Molarity = mol / L, [H2] = 0.2414 M, [N2] = .0804 M and [NH3] = 0.00544 M .
Plugging in to the mass action expression and solving for Q,
In the reaction quotient equation Q < Keq which means that in order to regain equilibrium, the
product must increase and the reactant decrease, a shift to the right in the overall reaction.
21
LeChatlier and Chemical Direction
January 13
Summary of Pressure, Temperature
Affect on Solubility
23
ΔH (s, l or g)
Temp
Direction
Solubility
(+) Endothermic
h
g Prod
h increase
(+) Endothermic
i
f React
i decrease
(-) Exothermic
h
f React
i decrease
(-) Exothermic
i
g Prod
h increase
Pressure
Direction
Solubility
Gas solute
h
g Prod
h increase
Gas solute
i
f React
i decrease
LeChatlier and Chemical Direction
January 13
Example Summary
Consider the following system at equilibrium:
6H2O (g) + 6CO2 (g) D 2 C6H12O6 (s) + 6O2 (g)
Complete the following table. Indicate changes in moles and concentrations by
entering I, D, N, or ? in the table.
(I = increase, D = decrease, N = no change, ? = insufficient information to determine)
24
LeChatlier and Chemical Direction
January 13
14.4 (Chp16) Solubility and
Solubility Product
What drives substances to dissolve and others to
remain as a precipitate?
Dr. Fred Omega Garces
Chemistry 201
Miramar College
1
Solubility and Solubility Product
January 13
When a Substance Dissolve
When a reaction occurs in which an insoluble product is
produced, the Keq is called a solubility-product.
BaSO4
(s)
D
Ba+2 + SO42-
Keq = Ksp = [Ba+2] [SO42-]
(aq)
Do not confuse
solubility (s)
with solubility
product Ksp.
Picture of BaSO4 dissolving
Solubility depends on Temp., conc., and pH
Solubility Product depends on Temp. Only !!!
2
Solubility and Solubility Product
January 13
Solubility
Solubility: What is the meaning of solubility ?
i.
The ability of substance to dissolve in a solvent.
ii. Quantity that dissolves to form a saturated solution.
s
g per 100 mL or g /L
g
(molar solubility, grams per liter saturated solution.)
g • mol
g
g
mol g Molarity
The greater the
solubility, the smaller
the amount of
precipitation.
3
Solubility and Solubility Product
January 13
Factors Affecting Solubility (s)
1 Nature of Solute (Concentration). - Like dissolves like (IMF)
2 Temperature Factor i) Solids/Liquids- Solubility increases with Temperature
Increase K.E. increases motion and collision between solute / solvent.
ii) gas - Solubility decreases with Temperature
Increase K.E. result in gas escaping to atmosphere.
3 Pressure Factor i) Solids/Liquids - Very little effect
Solids and Liquids are already lose together, extra pressure will not
increase solubility.
ii) Gas- Solubility increases with Pressure.
Increase pressure squeezes gas solute into solvent.
4
Solubility and Solubility Product
January 13
In a Chemical Reaction For a reaction to take place Reactants should be soluble in solventThis ensures that reactants combine (come in contact) with each
other efficiently.
When reaction proceedsProducts which are formed have different properties than that of
the reactants. One such property is the solubility.
When a precipitate forms upon mixing two solution it
is a result of a new specie that is present in the
solution.
5
Solubility and Solubility Product
January 13
Precipitation Reaction
Consider the reaction i AgNO 3 (aq) + HCl
(aq)
D
AgCl(s) + HNO3(aq)
(aq)
D
AgCl
Net ionic equation:
Ag+(aq) + Cl-
If written in this
form, then mass
action is:
(s)
Write the reverse, (standard convention)
AgCl
Then
(s)
D
Ag+(aq) + Cl-
(aq)
K eq =
Keq = Ksp = [Ag+] [Cl-]
1
[Ag + ][Cl− ]
Ksp - solubility product constant
An indicator of the solubility of substance of interest.
€
Yields info. on the amount of ions allowed in solution.
(Must be determine experimentally)
NaOCH2CH3 (s) + H2O (l) D Na+ (aq) + OH- (aq) + CH3CH2OH (aq)
6
Solubility and Solubility Product
Ksp = [Na+] [OH-] [CH3CH2OH]
January 13
Solubility Equilibrium: Example
What is the solubility product for the following reaction:
D 3Ag+ (aq) + PO43- (aq)
s = 4.4•10-5M
Ag3PO4
i
Excess
Δ
- 4.4•10-5M
3(4.4•10-5M )
Solid
1.32•10-4M
e
7
0
0
+ 4.4•10-5M
4.4•10-5 M
Solubility and Solubility Product
January 13
Solubility from Solubility Chart
The diagram below shows the solubility of some salts as a function of temperature.
This graph can be used to determine the Ksp for any one of the salts shown. Consider
KNO3, the solubility of KNO3 at 20°C according to the graph is approximately 30 g
per 100ml H2O. The strategy is to find the molar concentration and then use this
information to determine the Ksp for the salt at the specified temperature.
The solubility of KNO3 at 20°C is 30 g per
100ml H2O. What is the Ksp for KNO3 at
this temperature? Assume density of
solution = 1.0 g / cc
MWKNO3 = 101.11 g/mol
9
Solubility and Solubility Product
January 13
Solubility Vs. Ksp
Consider ;
La (IO3)3
Ksp = 6.2 • 10-12
11
<
Ba(IO3)2
Ksp = 1.5 • 10-9
Solubility and Solubility Product
<
AgIO3
Ksp = 3.1 •10-8
January 13
Precipitation and Common ion Effect
How can one predict if a precipitate forms when mixing solutions?
Solubility rules Soluble Substances
containing
Exceptions
Insoluble substances
containing
Exceptions
nitrates, (NO3-)
chlorates (ClO3-)
perchlorates (ClO4-)
acetates (CH3COO-)
None
carbonates (CO32-)
phosphates (PO43-)
Slightly soluble
halogens (X-)
X- = Cl-, Br-, I-
Ag, Hg, Pb
hydroxides (OH-)
alkali, NH4+, Ca,
Sr, Ba
sulfates (SO42-)
Ba, Hg, Pb
alkali & NH4+
None
Solubility rule provides information on the specie that will form a precipitate.
In general, a molar solubility of 0.01M or greater for a substance is
considered soluble.
http://www.ausetute.com.au/solrules.html
13
Solubility and Solubility Product
January 13
Precipitation Experiment
Consider the reaction:
3 Ca2+ + 2PO43-
(aq)
D Ca3(PO4)2
(s)
According to the solubility rules, this should form a precipitate (ppt) .
The question is what concentration is required before a precipitate forms ?
To solve,
It is always convenient to write the equation as a dissolution of solid
Ca3(PO4)2
(s)
D
3 Ca2+ + 2PO43-
Q = [Ca+2]3 • [PO43-]2
(aq)
next compare Q to Ksp
In solubility product problems,
Q is the ion-product instead of reaction quotient
14
Solubility and Solubility Product
January 13
Ion Product; Q
Q - indicator of direction of reaction for reaction not at equilb.
Direction
Ksp
Reaction
Q (i)
Unsaturated.
Q is small, system
consist mostly of ion.
No ppt. forms
(s) g (aq)
15
Q
Sat.
Point
Solubility and Solubility Product
Direction
Reaction
Q (h)
Supersaturated.
Q is large, system will
adjust to reduce high
ion concentration.
Solid forms
(s) f (aq)
January 13
In Class: Precipitation Determination
Question, (19.88 Sil): Will any solid Ba(IO3)2 form when 6.5 mg of BaCl2 is
dissolved in 0.500L of 0.033M NaIO3? ([Ba+2] = 6.2e-5 M)
Ksp Ba(IO3)2 = 1.5 •10-9 , BaCl2 MW = 208.23 g/mol
Ba(IO3)2 = Ba(IO3)2
16
(s)
D
Ba2+(aq) + 2 IO3-
Solubility and Solubility Product
(aq)
January 13
Common Ion Effect
For a system containing a precipitate in equilibrium with its ions, solubility
of solid can be reduced if an ionic compound is dissolved in the solution.
Ag+ I-
Ag+ I-
AgI(s)
AgI(s)
Ag+ I-
Add Ivia NaI
AgI(s)
AgI(s) D Ag+(aq) + I-(aq)
add common ion i.e.,
NaI to solution
Direction of Reaction
Reduce solubility
i.e., less AgI will dissolve in soln’ which
means more ppt. forms in solution
18
Solubility and Solubility Product
January 13
...according to LeChatelier
AgI(s)
Ag+
I-
AgI(s) D Ag+ + IQ analogy:
AgI(s)
NaI(s)
Ag+
AgI(s)
Q = [Ag+] [I-]
Ag+
I-
Ksp < Q
I-
Ksp
More AgI forms and
Solubility is lowered
19
Increase I-
Q
Direction of Rxn
(ppt occur)
Solubility and Solubility Product
January 13
Factor Affecting Solubility: Solubility & Common Ion
Calculate the solubility of copper(I) iodide, CuI (Ksp = 1.1•10-12)
a) water
b) 0.05 M sodium iodide
a)
20
i
Δ
e
CuI (s) D
Lots
-s
Solid
Cu+(aq) + I- (aq)
0
0
+s
+s
+s
+s
b) i
Δ
e
Solubility and Solubility Product
CuI (s) D
Lots
-s
Solid
Cu+(aq) +
0
+s
+s
I- (aq)
0.05
+s
0.05 + s
January 13
Factor Affecting Solubility: Solubility & Common Ion
Calculate the solubility of copper(I) iodide, CuI (Ksp = 1.1•10-12)
a) water
b) 0.05 M sodium iodide
a)
i
Δ
e
CuI (s) D
Lots
-s
Solid
Cu+(aq) + I- (aq)
0
0
+s
+s
+s
+s
b) i
Δ
e
CuI (s) D Cu+(aq) + I- (aq)
Lots
-s
Solid
0
+s
+s
0.05
+s
0.05 + s
a) Ksp = 1.1⋅10 -12 =[Cu+ ] [I - ] = s2
1.05⋅10 -6 = s
Without common ion, solubility is, s = 1.05 •10-6 M
21
Solubility and Solubility Product
January 13
Calculation: Solubility, Iteration Method
Reger Ex 12.19: What is the solubility of calcium fluoride in 0.025 M sodium
fluoride solution? Ksp = 6.2•10-8 M
i
Δ
e
23
CaF2 (s) D Ca+2(aq) + 2F- (aq)
Lots
0
0.025M
-s
+s
+2s
Solid
+s
0.025M +2s
Solubility and Solubility Product
January 13
Calculation: Solubility of Two salts in same solution
At 50°C, the solubility products, Ksp, of PbSO4 and SrSO4 are
1.6•10-8 M and 2.8•10-7M, respectively. What are the values of
[SO42-], [Pb2+], and [Sr2+] in a solution at equilibrium with both
substances ?
PbSO4 (s) D Pb2+(aq) + SO42 -(aq) ; Ksp = 1.6•10-8 = [Pb2+][SO42-]
SrSO4 (s) D Sr+ (aq) + SO4 2-(aq) ; Ksp = 2.8•10-7 = [Sr2+][SO42-]
Let x = [Pb2+] , y = [Sr2+] , x + y = [SO42-]
25
Solubility and Solubility Product
January 13
Calculation: Same type problem different data
At 25°C, the solubility product of AgI and PbI2 is 8.3•10-17 M and
7.9•10-9M, respectively. What are the values of [I-], [Ag+], and [Pb2+] in a
solution at equilibrium with both substances ? (Answer behind)
AgI
i Lots
Δ -s
e Lots
(s)
D Ag + (aq) +
0
+s
+s
I-(aq)
PbI2
i Lots
Δ -t
e Lots
2t
+s
+s+2t
K sp = [Ag+ ] ∗ [I -]
[I ] =
-
7.9 ⋅ 10-9
8.3 ⋅ 10-17
€
=
[Pb+2][I- ]2
[Ag+ ] [I- ]
=
[I -]2 =
[Ag+ ]
[s] [s+2t]
= 9.518 •107
€
[Pb+2][I- ]2 = 7.9 ⋅ 10-9 =[t] [s+2t]2 Assume s << t
7.9 ⋅ 10-9 = [t] [2t]2 = 4[t]3
28
Pb
D
0
+t
+t
+2
(aq)
+
2I- (aq)
s
+2t
s+2t
K sp = [Pb+2 ] ∗ [I -]2
K sp
[t] [s+2t]2
(s)
; t =1.25 •10−3 = [Pb+2]
K sp
[Pb+2 ]
[Ag+ ] [I- ] = 8.3 ⋅ 10-17 = [s] [s+t] Assume s << t
8.3 ⋅ 10-17 = [s] [t] = [s] [2.50 •10−3] ; [s] = 3.32 •10−14 M
[Ag+ ]=3.32 •10−14 M, [Pb+2] =1.25 •10−3 M, [I- ] =2.5 •10−3 M
Solubility and Solubility Product
January 13
Calculation: Same type problem different data
At 25°C, the solubility product of AgI and Hg2I2 is 8.5•10-17 M and
5.3•10-29M, respectively. What are the values of [I-], [Ag+], and [Hg22+] in
a solution at equilibrium with both substances ?
29
Solubility and Solubility Product
January 13
Common Ion Effect
B&L 17.44 (7th Ed.): The Ksp for cerium iodate, Ce(IO3)3, is 3.2 •10-10
a) Calculate the molar solubility of Ce(IO3)3 in pure water.
b) What concentration of NaIO3 in solution would be necessary to
reduce the Ce3+ concentration in a saturated solution of Ce(IO3)3 by a
factor of 10 below that calculation in part (a).
i
Δ
e
30
Ce(IO3)3
Lots
-s
Lots
(s)
D Ce+3(aq) +
0
+s
+s
3 IO3- (aq)
0
+3s
+3s
Solubility and Solubility Product
January 13
Qualitative Analysis
Ions are precipitated selectively by adding a
precipitation ion until the Ksp of one compound
is exceeded without exceeding the Ksp of the
others. An extension of this approach is to
control the equilibrium of the slightly soluble
compound by simultaneously controlling an
equilibrium system that contains the
precipitating ion. Qualitative analysis of ion
mixtures involves adding precipitating ions to
separate the unknown ions into ion groups. The
groups are then analyzed further through
precipitation and complex ion formation.
32
Solubility and Solubility Product
January 13
Selective Precipitation
Qualitative Analysis
Q < Ksp, solid dissolve until Q=Ksp
Q = Ksp, equilib
Q > Ksp, ppt until Q=Ksp
Grp1: Insoluble Chlorides
Ag+, Pb2+, Hg22+
Grp2: Acid-Insoluble sulfides
Cu2+, Bi3+, Cd2+, Pb2+, Hg2+, As3+, Sb3+, Sn4+
Grp3: Base-Insoluble sulfides
Cu2+, Al3+, Fe2+, Fe3+, Co2+, Ni2+, Cr3+, Zn2+, Mn2+
Grp4: Insoluble Phosphates
Ba2+, Ca2+, Mg2+
Grp5: Alkali Metals and NH4+
Na+, K+, NH4+
33
Solubility and Solubility Product
January 13
Lab Practical, Qualitative Analysis
Qualitative Analysis of Household Chemicals
34
Solubility and Solubility Product
January 13
Complex Ion Process
Metals forming Complexes: Metals by virtue of electron pairs will act as Lewis
acids and bind a substrate to form a Complex Consider the following:
Ag(NH3)2+ (aq) Complex ion
AgCl(s) D Ag+(aq) + Cl- (aq)
Ag+(aq) + 2NH3(aq) D Ag(NH3)2+ (aq)
AgCl(s) + 2NH3(aq) D Ag(NH3)2+ (aq) + Cl- (aq)
Normally insoluble AgCl can be made soluble
By the addition of NH3. The presence of NH3
drives the top reaction to the right and
increase the solubility of AgCl
An assembly of metal ion and the Lewis base (NH3)
is called a complex ion.
The formation of this complex is describe by
Kf =
Formation Constant, Kf
Ag+(aq) + 2NH3(aq) D Ag(NH3)2+ (aq)
[Ag(NH3 )2+ ]
[Ag+ ] ∗[NH3 ]2
= 1.7 • 10 7
Solubility of metal salts affected presence of Lewis Base: e.g., NH3, CN-, OH37
€
Solubility and Solubility Product
January 13
Formation Constants Table
38
Complex
Kf
[Ag(CN)2]–
Complex
Kf
5.6e18
[Cr(OH)4]–
[Ag(EDTA)]3–
2.1e7
[Ag(en)2]+
Complex
Kf
8e29
[HgI4]2–
6.8e29
[CuCl3]2–
5e5
[Hg(ox)2]2–
9.5e6
5.0e7
[Cu(CN)2]–
1.0e16
[Ni(CN)4]2–
2e31
[Ag(NH3)2]+
1.6e7
[Cu(CN)4]3–
2.0e30
[Ni(EDTA)]2–
3.6e18
[Ag(SCN)4]3–
1.2e10
[Cu(EDTA)]2–
5e18
[Ni(en)3]2+
2.1e18
[Ag(S2O3)2]3–
1.7e13
[Cu(en)2]2+
1e20
[Ni(NH3)6]2+
5.5e8
[Al(EDTA)]–
1.3e16
[Cu(CN)4]2–
1e25
[Ni(ox)3]4–
3e8
[Al(OH)4]–
1.1e33
[Cu(NH3)4]2+
1.1e13
[PbCl3]–
2.4e1
[Al(Ox)3]3–
2e16
[Cu(ox)2]2–
3e8
[Pb(EDTA)]2–
2e18
[Cd(CN)4]2–
6.0e18
[Fe(CN)6]4–
1e37
[PbI4]2–
3.0e4
[Cd(en)3]2+
1.2e12
[Fe(EDTA)]2–
2.1e14
[Pb(OH)3]–
3.8e14
[Cd(NH3)4]2+
1.3e7
[Fe(en)3]2+
5.0e9
[Pb(ox)2]2–
3.5e6
[Co(EDTA)]2–
2.0e16
[Fe(ox)3]4–
1.7e5
[Pb(S2O3)3]4–
2.2e6
[Co(en)3]2+
8.7e13
[Fe(CN)6]3–
1e42
[PtCl4]2–
1e16
[Co(NH3)6]2+
1.3e5
[Fe(EDTA)]–
1.7e24
[Pt(NH3)6]2+
2e35
[Co(ox)3]4–
5e9
[Fe(ox)3]3–
2e20
[Zn(CN)4]2–
1e18
[Co(SCN)4]2–
1.0e3
[Fe(SCN)]2+
8.9e2
[Zn(EDTA)]2–
3e16
[Co(EDTA)]–
1e36
[HgCl4]2–
1.2e15
[Zn(en)3]2+
1.3e14
[Co(en)3]3+
4.9e48
[Hg(CN)4]2–
3e41
[Zn(NH3)4]2+
4.1e8
[Co(NH3)6]3+
4.5e33
[Hg(EDTA)]2–
6.3e21
[Zn(OH)4]2–
4.6e17
[Co(ox)3]3–
1e20
[Hg(en)2]2+
2e23
[Zn(ox)3]4–
1.4e8
[Cr(EDTA)]–
1e23
Solubility and Solubility Product
January 13
Calculations: Formation of Complex Ion
By using the values of Ksp for AgI and Kf for Ag(CN)2-, calculate the
equilibrium constant for the following reaction:
AgI(s) + 2CN-(aq) D Ag(CN)2- (aq) + I-(aq)
Note that this equation is the sum of
39
AgI (s) D Ag+(aq) + I -(aq) ;
Ksp = 8.3•10-17 = [Ag+][I -]
Ag+(aq) + 2CN-(aq) D Ag(CN)2-(aq) ;
Kf = 1.0•1021 = [Ag(CN)2-]/[Ag+][CN -]2
Solubility and Solubility Product
January 13
Calculations: Formation of Complex Ion
By using the values of Ksp for AgI and Kf for Ag(CN)2-, calculate the
equilibrium constant for the following reaction:
AgI(s) + 2CN-(aq) D Ag(CN)2- (aq) + I-(aq)
Note that this equation is the sum of
AgI (s) D Ag+(aq) + I -(aq) ;
Ksp = 8.3•10-17 = [Ag+][I -]
Ag+(aq) + 2CN-(aq) D Ag(CN)2-(aq) ;
Kf = 1.0•1021 = [Ag(CN)2-]/[Ag+][CN -]2
AgI(s) + 2CN-(aq) D Ag(CN)2- (aq) + I-(aq)
K = Ksp• Kf = ( [Ag+][I -] ) • ( [Ag(CN)2]/[Ag+][CN -]2 )
K = Ksp• Kf = 8.3•10-17 • 1.0•1021
K = 8.3•104
40
Solubility and Solubility Product
January 13