Chemistry - Sanskriti School
... Ideal gas equation. Kinetic molecular theory of Gases. Behaviour of real gases; Deviation from ideal behaviour, liquefaction of gases, critical temperature. Liquid State - Vapour pressure (qualitative idea only, no mathematical derivations). Unit VI: Thermodynamics Concepts of System, types of syste ...
... Ideal gas equation. Kinetic molecular theory of Gases. Behaviour of real gases; Deviation from ideal behaviour, liquefaction of gases, critical temperature. Liquid State - Vapour pressure (qualitative idea only, no mathematical derivations). Unit VI: Thermodynamics Concepts of System, types of syste ...
chemistry notes on the mole - lessons
... and hydrogen peroxide H2O2(l). Both of these compounds contain hydrogen and oxygen. The only difference between the two, is that there is one extra oxygen atom in the hydrogen peroxide. Even though this difference seems very small, it results in significant differences in the properties of both subs ...
... and hydrogen peroxide H2O2(l). Both of these compounds contain hydrogen and oxygen. The only difference between the two, is that there is one extra oxygen atom in the hydrogen peroxide. Even though this difference seems very small, it results in significant differences in the properties of both subs ...
C:\D\Books\Cambridge University Press\CUP Problems\Problems.wpd
... the Big Bang. What is the mass fraction of hydrogen in the universe? (Cf. mole fractions, p. 5.) 5. Why is the distribution of elements on the Earth different from that of Universe? Discuss the processes that gave rise to the Earth. 6. Which planets have a composition similar to Earth? Why? 7. Look ...
... the Big Bang. What is the mass fraction of hydrogen in the universe? (Cf. mole fractions, p. 5.) 5. Why is the distribution of elements on the Earth different from that of Universe? Discuss the processes that gave rise to the Earth. 6. Which planets have a composition similar to Earth? Why? 7. Look ...
Stoichiometry - MolesAvacado
... relationships that exist in chemical formulas and chemical reactions. In simpler terms, stoichiometry refers to the ratio of substances in molecules or chemical reactions. Possibly the most frequently asked question of a teacher is “Why do I need to know this?” In the case of stoichiometry it is imp ...
... relationships that exist in chemical formulas and chemical reactions. In simpler terms, stoichiometry refers to the ratio of substances in molecules or chemical reactions. Possibly the most frequently asked question of a teacher is “Why do I need to know this?” In the case of stoichiometry it is imp ...
Head-Gordon`s
... However, it has been known for three decades that the exact energy is in fact a functional of only the electron density (a function of only 3, rather than 3n, variables). The only catch is that the functional is not known. In section 4, I discuss density functional theory (DFT) which focuses on the ...
... However, it has been known for three decades that the exact energy is in fact a functional of only the electron density (a function of only 3, rather than 3n, variables). The only catch is that the functional is not known. In section 4, I discuss density functional theory (DFT) which focuses on the ...
File
... (D) 9 the liquid is equal to 760 mm Hg When a sample of oxygen gas in a closed (C) temperature at which the solid, liquid, and container of constant volume is heated until its vapor phases are all in equilibrium absolute temperature is doubled, which of the (D) temperature at which liquid and vapor ...
... (D) 9 the liquid is equal to 760 mm Hg When a sample of oxygen gas in a closed (C) temperature at which the solid, liquid, and container of constant volume is heated until its vapor phases are all in equilibrium absolute temperature is doubled, which of the (D) temperature at which liquid and vapor ...
Chapter 3
... Begin with what you are given. You have 4.8 mol H2 4.8 mol H2 x 2 mol NH3 = 3.2 mol NH3 3 mol H2 3.2 mol NH3 x 17.0 g NH3 = 54.4 g NH3 ...
... Begin with what you are given. You have 4.8 mol H2 4.8 mol H2 x 2 mol NH3 = 3.2 mol NH3 3 mol H2 3.2 mol NH3 x 17.0 g NH3 = 54.4 g NH3 ...
Calculations and Chemical Equations Atomic mass: Mass of an
... Chemical Reaction: Interaction between substances that results in one or more new substances being produced ...
... Chemical Reaction: Interaction between substances that results in one or more new substances being produced ...
BC Science 10 Workbook Answers
... 2. PCBs were used for industrial products, such as heat exchange fluids, paints, plastics, and lubricants for electrical transformers. 3. PCBs stay in the environment for a long time. Aquatic ecosystems and species that feed on aquatic organisms are especially sensitive to the effects of PCBs. PCBs ...
... 2. PCBs were used for industrial products, such as heat exchange fluids, paints, plastics, and lubricants for electrical transformers. 3. PCBs stay in the environment for a long time. Aquatic ecosystems and species that feed on aquatic organisms are especially sensitive to the effects of PCBs. PCBs ...
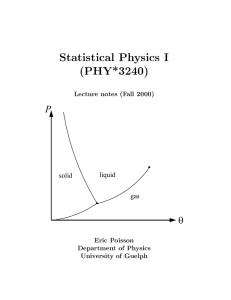
book - University of Guelph Physics
... Thermodynamics is the study of macroscopic systems for which thermal effects are important. These systems are normally assumed to be at equilibrium, or at least, close to equilibrium. Systems at equilibrium are easier to study, both experimentally and theoretically, because their physical properties ...
... Thermodynamics is the study of macroscopic systems for which thermal effects are important. These systems are normally assumed to be at equilibrium, or at least, close to equilibrium. Systems at equilibrium are easier to study, both experimentally and theoretically, because their physical properties ...
Dendrimer-Encapsulated Pd Nanoparticles as Aqueous, Room
... within each dendrimer. Reduction of G4-OH(Pd2+)40 with BH4results in zerovalent Pd DENs containing an average of 40 Pd atoms (G4-OH(Pd40)). The catalyst was initially tested for the Stille reaction using 4-iodobenzoic acid and phenyltin trichloride because both are soluble in basic aqueous solutions ...
... within each dendrimer. Reduction of G4-OH(Pd2+)40 with BH4results in zerovalent Pd DENs containing an average of 40 Pd atoms (G4-OH(Pd40)). The catalyst was initially tested for the Stille reaction using 4-iodobenzoic acid and phenyltin trichloride because both are soluble in basic aqueous solutions ...
PURPOSE: To determine the value of the equilibrium constant for a
... Lab 4 • Spectrophotometric Determination of an Equilibrium Constant PURPOSE: To determine the value of the equilibrium constant for a ...
... Lab 4 • Spectrophotometric Determination of an Equilibrium Constant PURPOSE: To determine the value of the equilibrium constant for a ...
honors chemistry harvard-westlake second semester final exam
... a. How many moles of gas have been collected? b. Write the chemical reaction, including the correct numerical energy term on the appropriate side c. Assuming that the zinc has been completely consumed, how many grams of zinc were used in this reaction? d. If the resulting solution was evaporated, wh ...
... a. How many moles of gas have been collected? b. Write the chemical reaction, including the correct numerical energy term on the appropriate side c. Assuming that the zinc has been completely consumed, how many grams of zinc were used in this reaction? d. If the resulting solution was evaporated, wh ...
SUPPLEMENTAL PROBLEMS FOR CHEM 110
... The enthalpy change for the reaction between Na(s) and H2O(g) to produce NaOH(s) and H2(g) is, per mole of H2(g) produced, A. B. C. D. E. ...
... The enthalpy change for the reaction between Na(s) and H2O(g) to produce NaOH(s) and H2(g) is, per mole of H2(g) produced, A. B. C. D. E. ...
The Complete Notes - Joliet Junior College
... remembering. An analogy would be this: you read all the books out there on the subject of golf, but don’t get round to swinging a club – what do you think happens when you tee off for the first time? ...
... remembering. An analogy would be this: you read all the books out there on the subject of golf, but don’t get round to swinging a club – what do you think happens when you tee off for the first time? ...
AS Specification pdf | AS/A level
... find arithmetic means by calculating relative atomic mass from mass spectrum data use ratios and percentages by solving empirical formula problems and calculating atom economy and yield of a reaction; recognise and make use of units in calculations involving amounts of substance; use powers in calcu ...
... find arithmetic means by calculating relative atomic mass from mass spectrum data use ratios and percentages by solving empirical formula problems and calculating atom economy and yield of a reaction; recognise and make use of units in calculations involving amounts of substance; use powers in calcu ...
Chemistry
... particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique atoms and the atoms of an element are all the same. At that time, there were about 35 known elements. T ...
... particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique atoms and the atoms of an element are all the same. At that time, there were about 35 known elements. T ...
Chemistry Review 2 answer key
... 'see explanation below' 24. Base your answer on the information below. Aluminum is one of the most abundant metals in Earth's crust. The aluminum compound found in bauxite ore is Al2O3. Over one hundred years ago, it was difficult and expensive to isolate aluminum from bauxite ore. In 1886, a brothe ...
... 'see explanation below' 24. Base your answer on the information below. Aluminum is one of the most abundant metals in Earth's crust. The aluminum compound found in bauxite ore is Al2O3. Over one hundred years ago, it was difficult and expensive to isolate aluminum from bauxite ore. In 1886, a brothe ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.