Conductometric and Potentiometric Determination of the Solubility
... of 0.01±0.001 M distigmine bromide (DsBr2) solution to 100 ml of each of the following: 0.0033±0.001 M PMA, 0.0033±0.001 M PTA, 0.0025±0.001 M SMA, 0.0025±0.001 M STA, 0.01±0.001 M NaTPB, 0.01±0.001 M AmmRein, and 0.01±0.001 M PiH. The obtained precipitates were washed thoroughly with distilled wate ...
... of 0.01±0.001 M distigmine bromide (DsBr2) solution to 100 ml of each of the following: 0.0033±0.001 M PMA, 0.0033±0.001 M PTA, 0.0025±0.001 M SMA, 0.0025±0.001 M STA, 0.01±0.001 M NaTPB, 0.01±0.001 M AmmRein, and 0.01±0.001 M PiH. The obtained precipitates were washed thoroughly with distilled wate ...
SCH3U Chemistry 11 Course Notes 2015
... for John Smith). Choose any appropriate password. Remember your password, a new one cannot be re-issued by your teacher! 4. Type in a valid email address, which you will be able to hide later if you go in & edit your profile. Enter your first name & last name in the appropriate fields. Follow the re ...
... for John Smith). Choose any appropriate password. Remember your password, a new one cannot be re-issued by your teacher! 4. Type in a valid email address, which you will be able to hide later if you go in & edit your profile. Enter your first name & last name in the appropriate fields. Follow the re ...
Thermochemistry
... Rules for using Hess’s law in solving problems 1. Make sure to rearrange the given equations so that reactants and products are on the appropriate sides of the arrows. 2. If you reverse equations, you must also reverse the sign of ΔH (i.e., if positive, change to negative) ...
... Rules for using Hess’s law in solving problems 1. Make sure to rearrange the given equations so that reactants and products are on the appropriate sides of the arrows. 2. If you reverse equations, you must also reverse the sign of ΔH (i.e., if positive, change to negative) ...
Homework Booklet [4,S]
... 2. Find the number of moles in each of these substances: (a) 284 g of sodium sulphate (b) 11 g of carbon dioxide (c) 1 kg of sodium hydroxide (d) 106 mg of sodium carbonate (e) 18.4 g of potassium sulphate (f) 11 kg of sulphur dioxide (g) 1x 10-6 g of potassium hydroxide (h) 210 mg of silver carbona ...
... 2. Find the number of moles in each of these substances: (a) 284 g of sodium sulphate (b) 11 g of carbon dioxide (c) 1 kg of sodium hydroxide (d) 106 mg of sodium carbonate (e) 18.4 g of potassium sulphate (f) 11 kg of sulphur dioxide (g) 1x 10-6 g of potassium hydroxide (h) 210 mg of silver carbona ...
IChO 2012
... temperature at which the two allotropes of tin are in equilibrium increase or decrease at that pressure, and by how much? In your quantitative calculations, you may assume that the energy (E), entropy (S), and molar volume of the two phases of tin are independent of temperature and pressure. ...
... temperature at which the two allotropes of tin are in equilibrium increase or decrease at that pressure, and by how much? In your quantitative calculations, you may assume that the energy (E), entropy (S), and molar volume of the two phases of tin are independent of temperature and pressure. ...
File
... Award [3] for correct final answer. Award [2] for (+)540. If old Data Booklet is used accept answer: –535 (kJ mol–1) or award [2] for (+)535. ...
... Award [3] for correct final answer. Award [2] for (+)540. If old Data Booklet is used accept answer: –535 (kJ mol–1) or award [2] for (+)535. ...
Click to open the TEOS-10 teaching aid slides(powerpoint)
... • The 1980 International Equation of State (EOS-80) has served the community very well for 30 years. • EOS-80 provides separate algorithms for density, sound speed, heat capacity and freezing temperature. • However, EOS-80 does not provide expressions for entropy, internal energy and most importantl ...
... • The 1980 International Equation of State (EOS-80) has served the community very well for 30 years. • EOS-80 provides separate algorithms for density, sound speed, heat capacity and freezing temperature. • However, EOS-80 does not provide expressions for entropy, internal energy and most importantl ...
Intelligent packaging Smart packaging
... product quality and value 1. Freshness indicators and indicators of microbial quality. • Principle – interaction between food and indicator. • The resources for this interaction can be various substances of food e.g. glucose, CO2 , ammonia, DMA and TMA, biogenic amines, sulphuric compounds, ethanol, ...
... product quality and value 1. Freshness indicators and indicators of microbial quality. • Principle – interaction between food and indicator. • The resources for this interaction can be various substances of food e.g. glucose, CO2 , ammonia, DMA and TMA, biogenic amines, sulphuric compounds, ethanol, ...
B.Sc. (CHEMISTRY) - Dr B. R. Ambedkar University
... Inorganic mixture analysis (preferably by semimicro method) – The mixture will have six ions, preferably three cations and three anions. It may contain ions of the same group and an interfering anion such as phosphate, oxalate, borate and fluoride. Not more than one interfering anion is to be given. ...
... Inorganic mixture analysis (preferably by semimicro method) – The mixture will have six ions, preferably three cations and three anions. It may contain ions of the same group and an interfering anion such as phosphate, oxalate, borate and fluoride. Not more than one interfering anion is to be given. ...
physical setting chemistry
... Sulfur dioxide, SO2, is one gas produced when fossil fuels are burned. When this gas reacts with water in the atmosphere, an acid is produced forming acid rain. The pH of the water in a lake changes when acid rain collects in the lake. Two samples of the same rainwater are tested using two indicator ...
... Sulfur dioxide, SO2, is one gas produced when fossil fuels are burned. When this gas reacts with water in the atmosphere, an acid is produced forming acid rain. The pH of the water in a lake changes when acid rain collects in the lake. Two samples of the same rainwater are tested using two indicator ...
5/14/01 - Oklahoma State University
... Although technology is beginning to make inroads into General Chemistry instruction, it has done little to change its nature. For the most part, technology is being used to supplement traditional instruction or to replicate traditional methods. We propose to use technology in a more central role Thi ...
... Although technology is beginning to make inroads into General Chemistry instruction, it has done little to change its nature. For the most part, technology is being used to supplement traditional instruction or to replicate traditional methods. We propose to use technology in a more central role Thi ...
Thermodynamics Theory + Questions.0001
... Fig. A quasi q – static process one the pressure of the gass will displace the piston gradually. It is quasista atic. • On the other hand if we remove all a the weights at once th he piston will be kicked up p by the gas pressure. (This is un nrestrained expansion) but b we don’tt consider th hat th ...
... Fig. A quasi q – static process one the pressure of the gass will displace the piston gradually. It is quasista atic. • On the other hand if we remove all a the weights at once th he piston will be kicked up p by the gas pressure. (This is un nrestrained expansion) but b we don’tt consider th hat th ...
Chapter Six - La Salle University
... ► The numbers and kinds of atoms must be the same on both sides of the reaction arrow. ► Numbers in front of formulas are called coefficients; they multiply all the atoms in a formula. ► The symbol 2 NaHCO3 indicates two units of sodium bicarbonate, which contains 2 Na, 2 H, 2 C, and 6 O. ► Substan ...
... ► The numbers and kinds of atoms must be the same on both sides of the reaction arrow. ► Numbers in front of formulas are called coefficients; they multiply all the atoms in a formula. ► The symbol 2 NaHCO3 indicates two units of sodium bicarbonate, which contains 2 Na, 2 H, 2 C, and 6 O. ► Substan ...
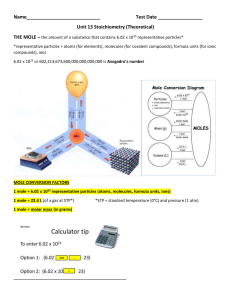
Unit 13 Stoichiometry (Theoretical)
... e. How many moles of Al2O3 are formed when 0.78 moles of O2 reacts with aluminum? (Ans. 0.52 mol Al2O3) f. How many grams of aluminum are required to produce 98.6 grams of aluminum oxide? ...
... e. How many moles of Al2O3 are formed when 0.78 moles of O2 reacts with aluminum? (Ans. 0.52 mol Al2O3) f. How many grams of aluminum are required to produce 98.6 grams of aluminum oxide? ...
Solution-Solubility-Equilibrium
... Hence, products can reform reactants. Essentially all chemical reactions are potentially reversible: i.e., once product particles are formed, these particles in turn can reform reactant particles. If product-product particle collisions do not result in significant particle rearrangements, then the e ...
... Hence, products can reform reactants. Essentially all chemical reactions are potentially reversible: i.e., once product particles are formed, these particles in turn can reform reactant particles. If product-product particle collisions do not result in significant particle rearrangements, then the e ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.



![Homework Booklet [4,S]](http://s1.studyres.com/store/data/010355871_1-63c750e3d1b58eaaebbb3f5d45651c44-300x300.png)