midterm 2 exam for section 3 from 2015
... that the calorimeter and liquids were all at the same temperature initially. (i) Determine which, if either, of the reagents is present in excess. ...
... that the calorimeter and liquids were all at the same temperature initially. (i) Determine which, if either, of the reagents is present in excess. ...
Department of Science - Chemistry
... Calculate collision theory and TST rates for di-atomic reactions Visualize simple potential energy surfaces Define and use the following: state functions, heat, work, internal energy, first law of thermodynamics, enthalpy, heat capacities, heats of formation, Hess’s law, entropy, second law of therm ...
... Calculate collision theory and TST rates for di-atomic reactions Visualize simple potential energy surfaces Define and use the following: state functions, heat, work, internal energy, first law of thermodynamics, enthalpy, heat capacities, heats of formation, Hess’s law, entropy, second law of therm ...
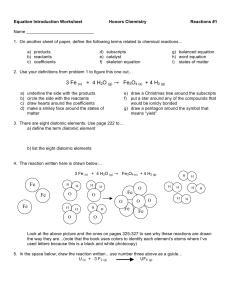
Balancing Chemical Equation Practice.docx
... Reading adapted from Sarquis’s Modern Chemistry Introduction A chemical reaction is the process by which one or more substances are changed into one or more different substances. In any chemical reaction, the original substances are known as the reactants, and the resulting substances are known as t ...
... Reading adapted from Sarquis’s Modern Chemistry Introduction A chemical reaction is the process by which one or more substances are changed into one or more different substances. In any chemical reaction, the original substances are known as the reactants, and the resulting substances are known as t ...
Lecture 5
... Suppose we add some thermal energy (dq) to a unit mass of a substance like air, water, soil. We expect T(substance) to increase ...
... Suppose we add some thermal energy (dq) to a unit mass of a substance like air, water, soil. We expect T(substance) to increase ...
Fluids – Lecture 11 Notes
... Introduction to Compressible Flows Definition and implications A compressible flow is a flow in which the fluid density ρ varies significantly within the flowfield. Therefore, ρ(x, y, z) must now be treated as a field variable rather than simply a constant. Typically, significant density variations start to ...
... Introduction to Compressible Flows Definition and implications A compressible flow is a flow in which the fluid density ρ varies significantly within the flowfield. Therefore, ρ(x, y, z) must now be treated as a field variable rather than simply a constant. Typically, significant density variations start to ...
Applied Physics - Revision World
... Boyle’s Law: pV = constant - Temperature remains constant (isothermal) - pV = constant and p1V1 = p2V2 - ΔU = 0 because the internal energy is dependent on temperature, which does not change - ΔQ = ΔW. If the gas expands to do work ΔW, & amount of heat ΔQ must be supplied - compression or expansion ...
... Boyle’s Law: pV = constant - Temperature remains constant (isothermal) - pV = constant and p1V1 = p2V2 - ΔU = 0 because the internal energy is dependent on temperature, which does not change - ΔQ = ΔW. If the gas expands to do work ΔW, & amount of heat ΔQ must be supplied - compression or expansion ...
Energy of Reactions
... You need to have known chemical reactions that you can combine to form the final chemical reaction You need the δH for each reaction If you need to reverse a reaction, you must also change the sign of δH If you need to multiply a reaction by a number, you must also multiply the δH When all reactions ...
... You need to have known chemical reactions that you can combine to form the final chemical reaction You need the δH for each reaction If you need to reverse a reaction, you must also change the sign of δH If you need to multiply a reaction by a number, you must also multiply the δH When all reactions ...
Concept of Energy
... Since the internal energy is fixed when one specifies the entropy and the volume, this relation is valid even if the change from one state of thermal equilibrium to another with infinitesimally larger entropy and volume happens in a non-quasistatic way (so during this change the system may be very ...
... Since the internal energy is fixed when one specifies the entropy and the volume, this relation is valid even if the change from one state of thermal equilibrium to another with infinitesimally larger entropy and volume happens in a non-quasistatic way (so during this change the system may be very ...
Chemistry and the material world
... Enthalpy favours the reaction. It is exothermic, heat is released. Entropy dis-favours the reaction. Order is increased, the number of particles decreases. The questions remains: Will the reaction occur spontaneously? ...
... Enthalpy favours the reaction. It is exothermic, heat is released. Entropy dis-favours the reaction. Order is increased, the number of particles decreases. The questions remains: Will the reaction occur spontaneously? ...
Origin of Order: Emergence and Evolution of Biological Organization
... On the other hand, thermodynamics is the field of physics that deals with the direction of flow, transformation, storage, dissipation of thermal energy (heat) and the interrelation between heat and other forms of energy. Thermodynamics is also concerned with systems of very large numbers of particle ...
... On the other hand, thermodynamics is the field of physics that deals with the direction of flow, transformation, storage, dissipation of thermal energy (heat) and the interrelation between heat and other forms of energy. Thermodynamics is also concerned with systems of very large numbers of particle ...
Thermodynamics
... Can be reported for any T. Use 298K unless otherwise indicated Hvapo:1 mole pure liquid vapourises to a gas at 1bar (+40.66 kJmol-1 at 373K for water) endothermic Hfuso:1mole pure solid melts to a pure liquid at 1bar (+6.01 kJmol-1 at 273K for ice) endothermic ...
... Can be reported for any T. Use 298K unless otherwise indicated Hvapo:1 mole pure liquid vapourises to a gas at 1bar (+40.66 kJmol-1 at 373K for water) endothermic Hfuso:1mole pure solid melts to a pure liquid at 1bar (+6.01 kJmol-1 at 273K for ice) endothermic ...
Classification of Matter
... Also the original solid (HgO) and the product (Hg) are not the same colour. HgO is red and Hg is shiny and silvery. We have gas escaping (as suggested by the loss in solid mass: 432 vs. 400g) and a solid that is different from the original (difference in colour); the combination of these two observ ...
... Also the original solid (HgO) and the product (Hg) are not the same colour. HgO is red and Hg is shiny and silvery. We have gas escaping (as suggested by the loss in solid mass: 432 vs. 400g) and a solid that is different from the original (difference in colour); the combination of these two observ ...
8th Grade Post Physical Science Test Study Guide PS 1: The
... 1. flashlight: chemical (battery) to electrical to radiant (light) and heat (thermal). 2. Toaster: electrical to mechanical to radiant to thermal 3. Microwave: electrical to mechanical to radiant to thermal and sound B. Mechanical, chemical, electrical energy, thermal, radiant and nuclear energy ...
... 1. flashlight: chemical (battery) to electrical to radiant (light) and heat (thermal). 2. Toaster: electrical to mechanical to radiant to thermal 3. Microwave: electrical to mechanical to radiant to thermal and sound B. Mechanical, chemical, electrical energy, thermal, radiant and nuclear energy ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.