Physical and Chemical Changes
... Hardness: Compare hardness of objects by seeing which one would scratch the other. ...
... Hardness: Compare hardness of objects by seeing which one would scratch the other. ...
Regents Chemistry Review Questions
... Under what conditions of temperature and pressure are real gases most like ideal gases? Under what conditions of temperature and pressure do gases deviate most from ideal? When an air bag in an automobile inflates, the nitrogen gas inside of it is at a pressure of 1.30 atmospheres, a temperature of ...
... Under what conditions of temperature and pressure are real gases most like ideal gases? Under what conditions of temperature and pressure do gases deviate most from ideal? When an air bag in an automobile inflates, the nitrogen gas inside of it is at a pressure of 1.30 atmospheres, a temperature of ...
The Chemical Bond
... B2, C2, O2, F2, Ne2, and Ne2+. Give the term symbol for the C2 molecule in its ground state, assuming its electronic configuration is…(2pπ)2; i.e., that there is an electron in each of the degenerate orbitals 2pπx and 2pπy. 9. Sketch the MO energy diagrams of CO, NO, and CN¯. Compare your results to ...
... B2, C2, O2, F2, Ne2, and Ne2+. Give the term symbol for the C2 molecule in its ground state, assuming its electronic configuration is…(2pπ)2; i.e., that there is an electron in each of the degenerate orbitals 2pπx and 2pπy. 9. Sketch the MO energy diagrams of CO, NO, and CN¯. Compare your results to ...
2-1 Checkpoint - Jordan High School
... – One end slightly positive (δ+) – Other end slightly negative (δ-) ...
... – One end slightly positive (δ+) – Other end slightly negative (δ-) ...
Chemical Equations
... • When hydrocarbons burn in a plentiful supply of oxygen, they give off heat to their surroundings and produce carbon dioxide and water • When limited air is available for a combustion reaction, carbon monoxide may be formed in preference to carbon ...
... • When hydrocarbons burn in a plentiful supply of oxygen, they give off heat to their surroundings and produce carbon dioxide and water • When limited air is available for a combustion reaction, carbon monoxide may be formed in preference to carbon ...
Chemical Reactions - hrsbstaff.ednet.ns.ca
... What is a chemical reaction? • A chemical reaction is a chemical change where chemical substances (called reactants) react to give new chemical substances (called products). • Example – The combustion of hydrogen in oxygen is a chemical reaction which gives water. • Hydrogen and Oxygen are the reac ...
... What is a chemical reaction? • A chemical reaction is a chemical change where chemical substances (called reactants) react to give new chemical substances (called products). • Example – The combustion of hydrogen in oxygen is a chemical reaction which gives water. • Hydrogen and Oxygen are the reac ...
Slide 1 - MrCard.Org
... given amt. of another material • catalyst – material that increases the rate of a reaction by lowering the activation energy • enzyme – a biological catalyst • inhibitor – material used to decrease rate of a reaction ...
... given amt. of another material • catalyst – material that increases the rate of a reaction by lowering the activation energy • enzyme – a biological catalyst • inhibitor – material used to decrease rate of a reaction ...
+ H 2 SO 4(aq) - Rothschild Science
... In any physical or chemical reaction, mass is neither created nor destroyed; it is conserved! Reactants Products Same number of atoms on both sides of the equation! ...
... In any physical or chemical reaction, mass is neither created nor destroyed; it is conserved! Reactants Products Same number of atoms on both sides of the equation! ...
Bagian 2 termodinamika
... collection of matter which has distinct boundaries. OR A real or imaginary portion of universe whish has distinct boundaries is called system. OR A thermodynamic system is that part of universe which is under thermodynamic study. ...
... collection of matter which has distinct boundaries. OR A real or imaginary portion of universe whish has distinct boundaries is called system. OR A thermodynamic system is that part of universe which is under thermodynamic study. ...
Answers to Final Exam Review
... covalent, and if ionic, write the formula for the predicted compound. a. Na and Fionic c. K and Brionic e. H and Ncovalent b. C and Ocovalent d. Ca and Fionic f. Mg and Oionic 33. Which statement describes the compound formed between sodium and oxygen? a. It is NaO2, which is ionic. c. It is Na2O, w ...
... covalent, and if ionic, write the formula for the predicted compound. a. Na and Fionic c. K and Brionic e. H and Ncovalent b. C and Ocovalent d. Ca and Fionic f. Mg and Oionic 33. Which statement describes the compound formed between sodium and oxygen? a. It is NaO2, which is ionic. c. It is Na2O, w ...
Chapter one
... measurement of the amount of a solution of known concentration that is required to react completely with a measured amount of a solution of unknown concentration. ...
... measurement of the amount of a solution of known concentration that is required to react completely with a measured amount of a solution of unknown concentration. ...
Slide 1
... In every balanced equation each side of the equation has the same number of atoms of each element ...
... In every balanced equation each side of the equation has the same number of atoms of each element ...
Ch. 1: Matter and Change
... They cannot be broken into anything else by physical or chemical means (one type of ...
... They cannot be broken into anything else by physical or chemical means (one type of ...
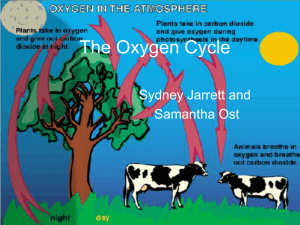
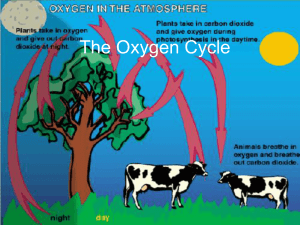
The Oxygen Cycle
... process in which solid rock exposed at earths surface is changed to separate solid particles and dissolved materials ...
... process in which solid rock exposed at earths surface is changed to separate solid particles and dissolved materials ...
Fundamental Concepts
... process in which there is heat transfer, it is represented with a straight line, PVn = constant. Throttling process - a process in which there is no change in enthalpy, no work is done and the process is ...
... process in which there is heat transfer, it is represented with a straight line, PVn = constant. Throttling process - a process in which there is no change in enthalpy, no work is done and the process is ...
Document
... Choose the correct answer :1- An endothermic reaction is one in which there is a. a positive value for the work (w > 0 joules b. a negative value for ΔH (ΔH < 0 joules) c. a positive value for ΔH (ΔH > 0 joules) d. a negative value for ΔE (ΔE > 0 joules) 2- For a change in a system taking place at ...
... Choose the correct answer :1- An endothermic reaction is one in which there is a. a positive value for the work (w > 0 joules b. a negative value for ΔH (ΔH < 0 joules) c. a positive value for ΔH (ΔH > 0 joules) d. a negative value for ΔE (ΔE > 0 joules) 2- For a change in a system taking place at ...
COUNTING ATOMS
... represents the number of each atom present. • Example: • H2 + O2 H20 • N2 + H2 NH3 ...
... represents the number of each atom present. • Example: • H2 + O2 H20 • N2 + H2 NH3 ...
What is a mixture?
... Distillation (boiling the solvent to separate it from the solute) 6. Magnetism (like iron) ...
... Distillation (boiling the solvent to separate it from the solute) 6. Magnetism (like iron) ...
Physical Biochemistry BIOL 4001 Fall Semester 2016 Term: August
... We may define biochemistry as the field of science comprising the knowledge of the properties of chemical reactions and physical processes also involving molecules of biological interest. Under this definition we can easily understand the importance of knowing basic physical principles, which ultima ...
... We may define biochemistry as the field of science comprising the knowledge of the properties of chemical reactions and physical processes also involving molecules of biological interest. Under this definition we can easily understand the importance of knowing basic physical principles, which ultima ...
CHEM MINI-COURSE SERIES M1.2___
... The one common feature of all chemical reactions is that they all produce new substances. These new substances have distinctly different properties when compared to the reactants, and they have new chemical formulas. In the laboratory, we may identify the new substances formed in a chemical reaction ...
... The one common feature of all chemical reactions is that they all produce new substances. These new substances have distinctly different properties when compared to the reactants, and they have new chemical formulas. In the laboratory, we may identify the new substances formed in a chemical reaction ...
Investigating Matter Notes
... A chemical change is a _____________________ change in matter that occurs when _____________ combine to form ______________ substances. We say a _____________________________ has been formed because the ___________________ are rearranged into different __________________________. ...
... A chemical change is a _____________________ change in matter that occurs when _____________ combine to form ______________ substances. We say a _____________________________ has been formed because the ___________________ are rearranged into different __________________________. ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.